Image by MethoxyRoxy – Own work, CC BY-SA 2.5
By: Neha Madugala, Neurology, Physiology, and Behavior, ‘21
Author’s Note: After writing a paper for the Aggie Transcript on the basics of dendritic spines, I wanted to take a more in-depth look at current research in this field by interviewing the UC Davis professor Karen Zito, who is actively involved in dendritic spine research. While there are still a lot of questions that remain unanswered within this field, I was interested in learning more about current theories and hypotheses that address some of these questions. Special thanks to Professor Zito for talking to us about her research. It was an honor to talk to her about her passion and knowledge for this exciting and complex field.
Preface: This interview is a follow-up to an original literature review on dendritic spines. For a more in-depth look at general information on dendritic spines, check out this article.
Neha Madugala (NM): Can you briefly describe some of the research that your lab does?
Dr. Karen Zito (KZ): My lab is interested in learning and memory. Specifically, we want to understand the molecular and cellular changes that occur in the brain as we learn. Our brain consists of circuits, connecting groups of neurons, and the function of these circuits allows us to learn new skills and form new memories. Notably, the strength of connections between specific neurons in these circuits can change during learning, or neurons can form new connections with other neurons to support learning. We have been mainly focusing on structural changes in the brain. This includes questions such as the following: How do neurons change in structure during learning? How do new circuit connections get made? What are the molecular signaling pathways that are activated to allow these changes to happen while learning? How is the plasticity of neural circuits altered with age or due to disease?
NM: What are dendritic spines?
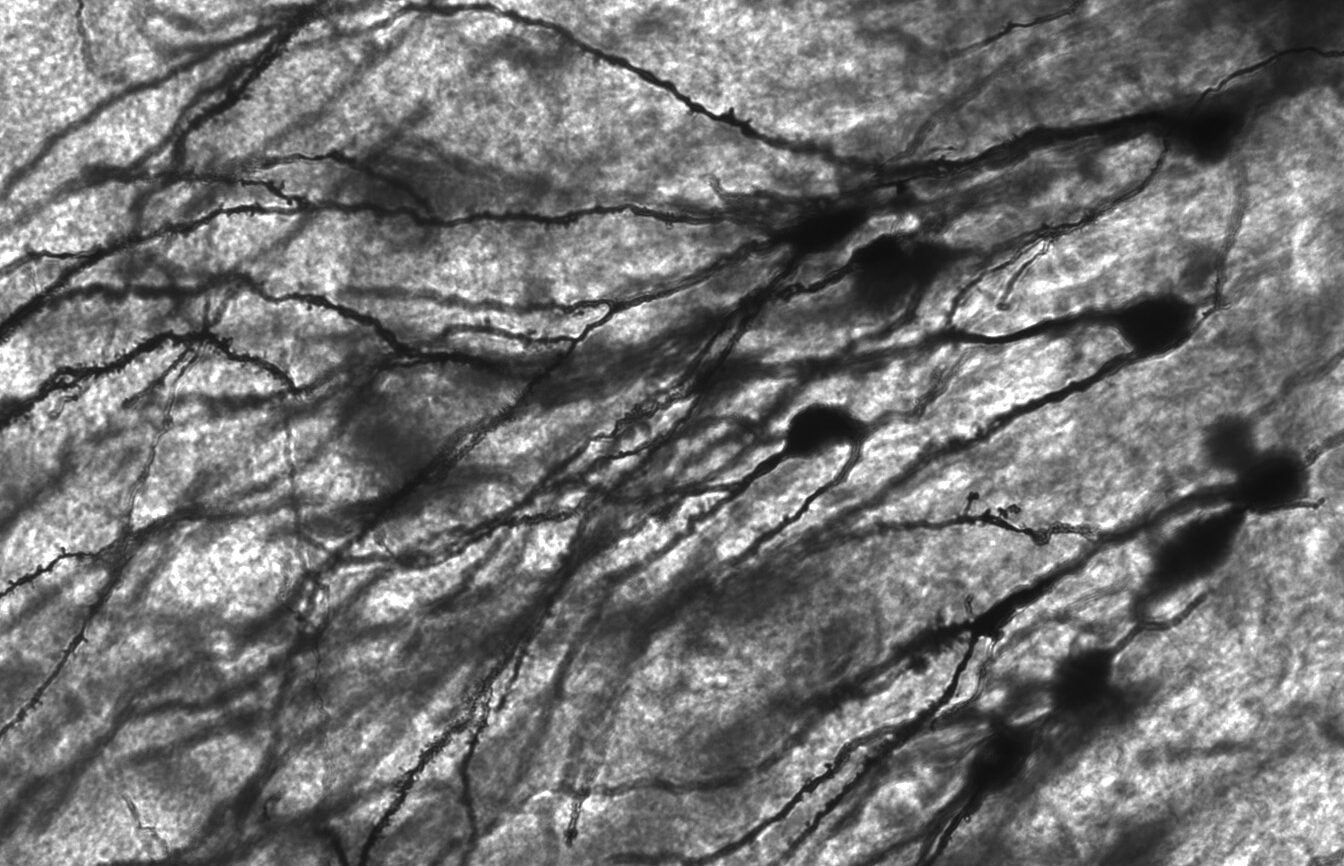
KZ: Dendritic spines are microscopic protrusions from dendrites of neurons and are often the site of change associated with learning. Axons of one neuron will synapse onto the dendritic spine of another neuron. Spines will grow and retract during development and synapses between a spine and axon will form during learning, forming complex circuits that allow us to do intricate tasks such as playing the piano.
NM: Transient spines only last a couple of days. What role do they play in learning?
KZ: One hypothesis for the function of transient spines is that they exist to sample the environment, allowing the brain to speed up its ability to find the right connections required for learning. Thus, the rapid growth and retraction of transient spines in the brain helps our neurons find the right connections required to form the new neural circuits by sampling many more connections and narrowing in on the right ones. For instance, in a past study on songbirds, researchers found that baby songbirds with faster moving transient spines were able to learn songs quicker than baby songbirds with slower moving transient spines. Once these transient spines find the right connection, they will transition from transient to a permanent spine to partake in a circuit that supports a new behavior, such as the songbird learning a new song.
NM: Can presynaptic neurons directly synapse onto a dendrite or only a dendritic spine?
KZ: Many neurons do not have spines at all. Spines are predominantly present on neurons in the higher order areas of the brain involved in learning, memory, perception and cognition. Spiny neurons are present in areas of the brain where neural connections are changing over time, or plastic — allowing the brain to learn, adjust, and change. Certain areas of the brain do not require a lot of change and, in some cases, circuit change may be detrimental to function. For example, we may not want to change connections established for movement of specific muscles.
NM: What is the difference between synapses that occur directly on a dendrite versus onto a dendritic spine?
KZ: Importantly, the molecular composition at synapses can vary widely between synapses, regardless of whether this connection occurs at a shaft or a spine. Therefore, it is difficult to name specific compositional elements always found at a spine versus a shaft. Inhibitory synapses, formed by GABAergic neurons, tend to be found directly on the shaft of dendrites. Glutamatergic neurons, which are excitatory, in the cerebral cortex tend to synapse on dendritic spines, but can also connect directly with the dendrite.
NM: All dendritic spines have excitatory synapses that require NMDA and AMPA receptors [1]. Are these receptors necessary for these spines to exist?
KZ: To my knowledge, we do not know the answer to this question. It is possible to remove these receptors a few at a time, and spines do not disappear. However, it is really hard to remove a receptor from a single spine and, if the receptors are removed from the entire neuron, it is often replaced with another receptor in a process called compensation. In order to test if this is possible, someone would have to knock out all genes encoding AMPA receptors and NMDA receptors, which is over seven genes, to see if spines still formed. Notably, if AMPA receptors are internalized, the spine typically shrinks, and if more AMPA receptors are brought to the surface, the spine typically grows. Indeed, the number of AMPA receptors at the synapse is directly proportional to the size of the spine.
NM: What drives spine formation and elimination when creating and refining neural circuits?
KZ: There really is no definitive answer to this question currently, and many of those performing dendritic spine research are interested in answering these questions. Let’s first look at formation. One theory suggests that there are factors coming from neighboring neurons, such as glutamate or BDNF [2], which promote spine formation. However, it is unclear which of these are acting in vivo, in the animal. Also, spine formation is much greater in younger animals compared to older animals. That can suggest that the cells are in a different state when younger versus older. The cell can be a less plastic state where all the spines are moving slowly, seen in older animals, or more plastic states where all the spines are moving more quickly, seen in younger animals. Thus, there appears to be a combination of intrinsic state, or how plastic the cell is, and extracellular factors such as the presence of glutamate that dictates spine formation. Elimination is similar in that we do not really know the entire molecular signaling sequence that is driving it. It is a fascinating question for so many reasons. For example, when we are young we overproduce spines, and as we grow the spine density declines as our nervous system selectively chooses which connections to keep. Then, as adults, our spine density remains relatively stable. However, we obviously keep learning as adults, even though our spine density remains constant. One hypothesis is that, as a spine grows while learning, a nearby spine with no activity shrinks and eventually becomes eliminated. In fact, we have observed this phenomenon in our studies. Therefore, there may be some local competition between these spines for space. This keeps the density the same across most of the adult life span.
NM: Does learning drive the formation of synaptic spines or does synaptic spine formation drive learning?
KZ: This may depend on the type of learning. Both have been observed. Studies have been done imaging the brain during learning. Some people have found an increase in new spine growth suggesting that learning drives new spine formation. Other people say they found the same number of new spine growth, but a greater amount of new spine stabilization, suggesting that learning drives new spine stabilization.
NM: It has been observed that some intellectual disabilities and neuropsychiatric disorders are associated with an abnormal number of dendritic spines when compared to a neurotypical individual. Is this related to the insufficient production of dendritic spines at birth or deficits in pruning?
KZ: Indeed autism spectrum disorders have been associated with an increase in spines. This could potentially be associated with an overproduction of spines or reduced spine elimination. Notably, the majority of neurological disorders resulting in cognitive deficits, such as Alzheimer’s disease, are associated with decreased spine densities. It is unclear if the spine numbers or brain function diminishes first, but much of the current research seems to suggest that the spines go away first, leading to the cognitive problems observed. In many disorders with too few spines, there is a normal formation of spines but excessive elimination. This is seen as Alzheimer and schizophrenic patients’ spine density is relatively normal prior to disease onset. For Alzheimer’s specifically, some researchers suggest that the molecular release of the pathogenic amyloid beta peptide binds to molecules on the surface of the dendritic spine that drive spine loss.
NM: How might dendritic spine research help in treating neuropsychiatric and neurodegenerative disorders?
KZ: Current research is looking at how to stabilize and destabilize dendritic spines. If we were able to manipulate the stability of these spines, we could potentially help rescue the stability of spines in patients with neuropsychiatric disorders, which could potentially lead to better therapies and outcomes. Understanding the pathways that control the stability of these spines will allow researchers to find targets for future therapeutic treatments.
Footnotes
- Receptors that are permeable to cations. They are usually associated with the depolarization of neurons.
- Brain-derived neurotrophic factor (BDNF): Plays a role in the growth and development of neurons.