Photo originally by MethoxyRoxy on Wikimedia Commons. No changes. CC License BY-SA 2.5.
By Neha Madugala, Cognitive Science, ‘21
Author’s Note: Last quarter I took Neurobiology (NPB100) with Karen Zito, a professor at UC Davis. I was interested in her research in dendritic spines and its correlation to my personal area of interest in research regarding the language and cognitive deficiencies present in different populations such as individuals with schizophrenia. There seems to be a correlational link between the generation and quantity of dendritic spines and the presence of different neurological disorders. Given the dynamic nature of dendritic spines, current research is studying their exact role and the potential to manipulate these spines in order to impact learning and memory.
Introduction
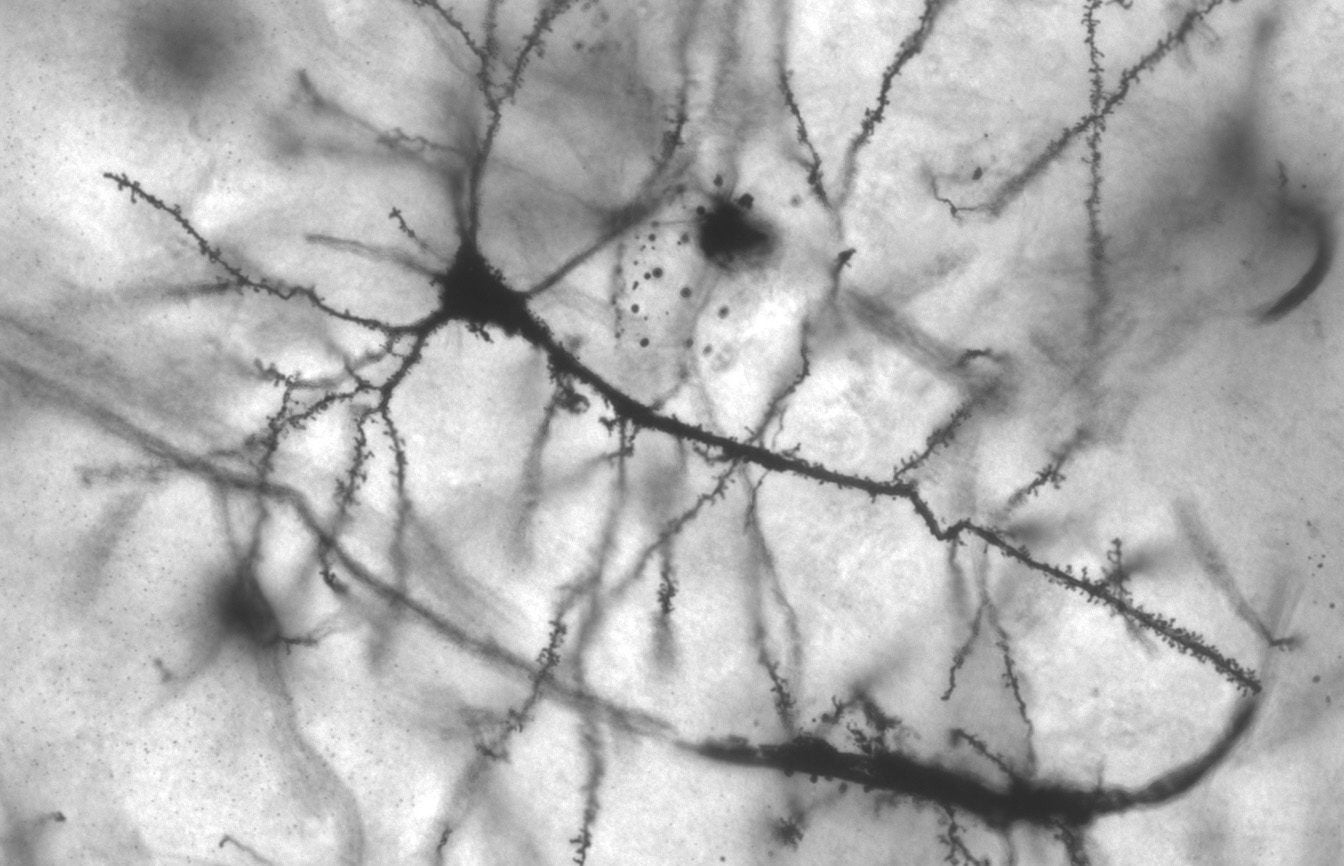
Dendritic spines are small bulbous protrusions that line the sides of dendrites on a neuron [12]. Dendritic spines serve as a major site of synapses for excitatory neurons, which continue signal propagation in the brain. Relatively little is known about the exact purpose and role of dendritic spines, but as of now, there seems to be a correlation between the concentration of dendritic spines and the presence of different disorders, such as autism spectrum disorders (ASD), schizophrenia, and Alzheimer’s disease. Scientists hypothesize that dendritic spines are a key player in the pathogenesis of various neuropsychiatric disorders [8]. It should be noted that other morphological changes are also observed when comparing individuals with the mentioned neuropsychiatric disorders are compared to neurotypical individuals. However, all these disorders share the common thread of abnormal dendritic spine density.
The main disorders studied in relation to dendritic spine density are autism spectrum disorder (ASD), schizophrenia, and Alzheimer’s disease. Current studies suggest that these disorders result in the number of dendritic spines straying from what is observed in a neurotypical individual. It should be noted that there is a general decline in dendritic spines as an individual ages. However intellectual disabilities and neuropsychiatric disorders seem to alter this density at a more extreme rate. The graph demonstrates the general trend of dendritic spine density for various disorders; however, these trends may slightly vary across individuals with the same disorder.
Dendritic Spines
I. Role of Dendritic Spines
Dendritic spines are protrusions found on certain types of neurons throughout the brain, such as in the cerebellum and cerebral cortex. They were first identified by Ramon y Cajal, who classified them as “thorns or short spines” located nonuniformly along the dendrite [6].
The entire human cerebral cortex consists of 1014 dendritic spines. A single dendrite can contain several hundred spines [12]. There is an overall greater density of dendritic spines on peripheral dendrites versus proximal dendrites and the cell body [3]. Their main role is to assist in synapse formation on dendrites.
Dendritic Spines fall into two categories: persistent and transient spines. Persistent spines are considered ‘memory’ spines, while transient spines are considered ‘learning’ spines. Transient spines are categorized as spines that exist for four days or less and persistent spines as spines that exist for eight days or longer [5].
The dense concentration of spines on dendrites is crucial to the fundamental nature of dendrites. At an excitatory synaptic cleft, the release of the neurotransmitter at excitatory receptors on the postsynaptic cell results in an excitatory postsynaptic potential (EPSP), which causes the cell to fire an action potential. An action potential is where a signal is transmitted from one neuron to another neuron. In order for a neuron to propagate an action potential, there must be an accumulation of positive charge at the synapses, reaching a certain threshold (Figure 2). The cell must reach a certain level of depolarization – a difference in charge across the neuron’s membrane making the inside more positive. A single EPSP may not result in enough depolarization to reach this action potential threshold. As a result, the presence of multiple dendritic spines on the dendrite allows for multiple synapses to be formed and multiple EPSPs to be summated. With the summation of various EPSPs on the dendrites of the neurons, the cell can reach the action potential threshold. The greater density of dendritic spines along the postsynaptic cell allows for more synaptic connections to be formed, increasing the chance of an action potential to occur.
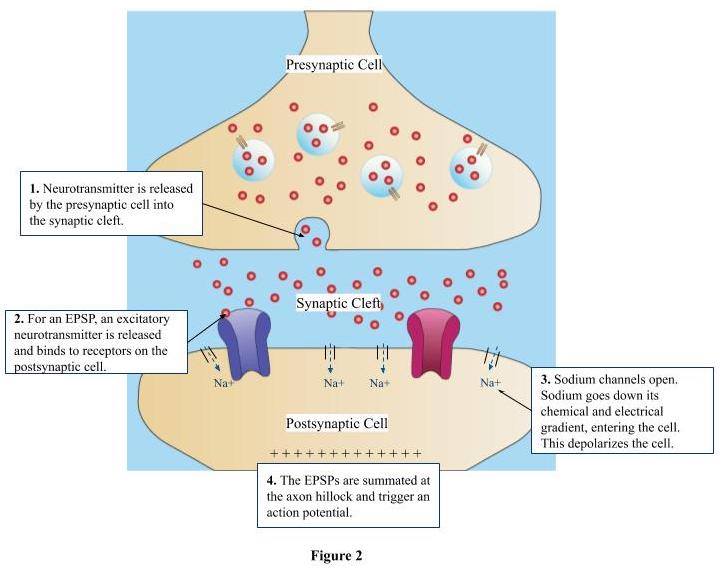
Figure 2. Firing of Action Potential (EPSP)
- Neurotransmitter is released by the presynaptic cell into the synaptic cleft.
- For an EPSP, an excitatory neurotransmitter will be released, which will bind to receptors on the postsynaptic cell.
- The binding of these excitatory neurotransmitters will result in sodium channels opening, allowing sodium to go down its electrical and chemical gradient – depolarizing the cell.
- The EPSPs will be summated at the axon hillock and trigger an action potential.
- This actional potential will cause the firing cell to release a neurotransmitter at its axon terminal, further conveying the electrical signal to other neurons.
II. Creation
Dendrites initially are formed without spines. As development progresses, the plasma membrane of the dendrite forms protrusions called filopodia. These filopodia then form synapses with axons, and eventually transition from filopodia to dendritic spines [6].
The reason behind the creation of dendritic spines is currently unknown. There are a few potential hypotheses. The first hypothesis suggests that the presence of dendritic spines can increase the packing density of synapses, allowing for more potential synapses to be formed. The second hypothesis suggests that their presence can help prevent excitotoxicity, overexcitation of the excitatory receptors (NMDA and AMPA receptors) present on the dendrites. These receptors usually bind with glutamate, a typically excitatory neurotransmitter, released from the presynaptic cell. This can result in damage to the neuron or if more severe, neuronal death. Since dendritic spines compartmentalize charge [3], this feature helps prevent the dendrite from being over-excited beyond the threshold potential for an action potential. Lastly, another hypothesis suggests that the large variation in dendritic spine morphology suggests that these different shapes play a role in modulating how postsynaptic potentials can be processed by the dendrite based on the function of the signal.
The creation of these dendritic spines is rapid during early development, slowly tapering off as the individual gets older. This process is mostly replaced with the pruning of synapses formed with dendritic spines when the individual is older. Pruning helps improve the signal-to-noise ratio of signals sent within neuronal circuits [3]. The signal-to-noise ratio outlines the ratio of signals sent by neurons and signals actually received by postsynaptic cells. It determines the efficiency of signal transmission. Experimentation has shown that the presence of glutamate and excitatory receptors (such as NMDA and AMPA) can result in the formation of dendritic spines within seconds [3]. The introduction of NMDA and AMPA results in cleavage of intracellular adhesion molecule-5 (ICAM5) from hippocampal neurons. ICAM5 is a “neuronal adhesion molecule that regulates dendritic elongation and spine maturation. [11]” Furthermore, through a combination of fluorescent dye and confocal or two-photon laser scanning microscopy, scientists were able to use imaging technology to witness that spines can undergo minor changes within seconds and more drastic conformational changes, even disappearing over minutes to hours [12].
III. Morphology
The spine head’s morphology, a large bulbous head connected to a very thin neck that attaches to the dendrite, assists in its role as a postsynaptic cell. This shape allows one synapse at a dendritic spine to be activated and strengthened without influencing neighboring synapses [12].
Dendritic spine shape is extremely dynamic, allowing one spine to slightly alter its morphology throughout its lifetime [5]. However, dendritic spine morphology seems to take on a predominant form that is determined by the brain region of its location. For instance, presynaptic neurons from the thalamus take on the mushroom shape, whereas the lateral nucleus of the amygdala have thin spines on their dendrites [2]. The type of neuron and brain region the spine originates from seem to be correlated to the observed morphology.
The spine contains a postsynaptic density, which consists of neurotransmitter receptors, ion channels, scaffolding proteins, and signaling molecules [12]. In addition to this, the spine has smooth endoplasmic reticulum, which forms stacks called spine apparatus. It further has polyribosomes, hypothesized to be the site of local protein synthesis in these spines, and an actin-based cytoskeleton for structure [12]. The actin-based cytoskeleton makes up for the lack of microtubules and intermediate filaments, which play a crucial role in the structure and transport of most of our animal cells. Furthermore, these spines are capable of compartmentalizing calcium, the ion used at neural synapses that signal the presynaptic cell to release its neurotransmitter into the synaptic cleft [12]. Calcium plays a crucial role in second messenger cascades, influencing neural plasticity [6]. It also plays a role in actin polymerization, which allows for the motile nature of spine morphology [6].
There are many various shapes for dendritic spines. The common types are ‘stubby’ (short and thick spines with no neck), ‘thin’ (small head and thin neck), ‘mushroom’ (large head with a constricted neck), and ‘branched’ (two heads branching from the same neck) [12].
IV. Learning and Memory
Dendritic spines play a crucial role in memory and learning through occurrence of long-term potentiation (LTP), which is thought to be the cellular level of learning and memory. LTP is thought to induce spine formation, which hints at the common correlation that learning is associated with the formation of dendritic spines. Furthermore, LTP is thought to be capable of altering the immature and mature hippocampus, commonly associated with memory [2]. To contrast LTP, long-term depression (LTD) essentially works opposite to LTP – decreasing the dendritic spine density and size [2].
The correlation between dendritic spines and learning is relatively unknown. There seems to be a general trend suggesting that the creation of these spines is associated with learning. However, it is unclear whether learning results in the formation of these spines or if the formation of these spines results in learning. The general idea behind this hypothesis is that dendritic spines aid in the formation of synapses, allowing the brain to form more connections. As a result, a decline in these dendritic spines in neuropsychiatric disorders, such as schizophrenia, can inhibit an individual’s ability to learn. This is observed in various cognitive and linguistic deficiencies observed in individuals with schizophrenia.
Memory is associated with the strengthening and weakening of connections due to LTP and LTD, respectively. The alteration of these spines through LTP and LTD is called activity-dependent plasticity [6]. The main morphological shapes associated with memory are the mushroom spine, a large head with a constricted neck, and the stubby spine, a short and thick spine with no neck [6]. Both of these spines are relatively large, resulting in more stable and enduring connections. These bigger and heavier spines associated with learning are a result of LTP. By contrast, transient spines (live four days or shorter) are usually smaller and more immature in morphology and function, resulting in more temporary and less stable connections.
LTP and LTD play a crucial role in modifying dendritic spine morphology. Neuropsychiatric disorders can alter these mechanisms resulting in abnormal density and size of these spines.
Schizophrenia
I. What is Schizophrenia?
Schizophrenia is a mental disorder that results in disordered thinking and behaviors, hallucinations, and delusions [9]. The exact mechanics of schizophrenia are still being studied as researchers are trying to determine the underlying biological reasons behind this disorder and a way to help these individuals. Current treatment is focused on reducing and in some cases treating symptoms of this disorder, but more research and understanding is required to fully treat this mental disorder.
II. Causation
The exact source of schizophrenia seems to lie somewhere between the presence of certain genes and environmental effects. There seems to be a correlation between traumatic or stressful life events during an individual’s adolescence to an increased susceptibility to developing schizophrenia [1]. While research is still underway, certain studies point to cannabis having a role in increasing susceptibility to schizophrenia or worsening symptoms if an individual already has schizophrenia [1]. There seems to be some form of a genetic correlation, given an increased likelihood of developing schizophrenia if present in a family member. This factor seems to result from a combination of genes; however, no genes have been identified yet. There also seems to be a chemical component, given the variation of chemical composition and density of neurotypical individuals and individuals with schizophrenia. Specifically, researchers have observed an elevated amount of dopamine found in individuals with schizophrenia [1].
III. Relationship between Dendritic Spines and Schizophrenia
A common thread among most schizophrenia patients is an impairment of pyramidal neuron (prominent cell form found in the cerebral cortex) dendritic morphology, occurring in various regions of the cerebral cortex [7]. Observed in postmortem brain tissue studies, there seems to be a reduced density of dendritic spines in the brains of individuals with schizophrenia. These findings are consistent with various regions of the brain that have been studied, such as the frontal and temporal neocortex, the primary visual cortex, and the subiculum within the hippocampal formation [7]. Out of seven studies observing this finding, the median reported decrease in spine density was 23%, with the overall range of these various studies being a decline of 6.5% to 66% [7].
It should be noted that studies were done to see if the decline in spine density was due to the usage of antipsychotic drugs. However animal and human trials showed no significant difference in the dendritic spine density of tested individuals.
This decline in dendritic spine density is hypothesized to be the result of the failure of the brain of schizophrenic individuals to produce sufficient dendritic spines at birth or if there is a more rapid decline of these spines during adolescence, where the onset of schizophrenia is typically observed [7]. The source of this decline is unclear, but seems to be attributed to deficits in pruning, maintenance, or simply the mechanisms of the underlying formation of these dendritic spines [7].
However, there are conflicting results. For instance, Thompson et al. conducted a study that seemed to suggest that a decline in spine density resulted in a progressive decline of gray matter, typically observed in schizophrenic individuals. Thompson et al. conducted an in vivo study of this phenomena. The study used MRI scans for twelve schizophrenic individuals and twelve neurotypical individuals, finding a progressive decline in gray matter – starting in the parietal lobe and expanding out to motor, temporal, and prefrontal areas [10]. The study suggests that the main attribution for this is a decline in dendritic spine density with the progression of the disorder. This study coincides with the previously mentioned hypothesis of a decline of spines during adolescence.
It is also possible that there is a combination of both of these factors occurring. Most studies have only been able to observe postmortem brain tissue, creating the confusion of whether there is a decline in spines or if the spines are simply not produced in the first place. The lack of in vivo studies makes it difficult to find a concrete trend within data.
Conclusion
While research is still ongoing, current evidence seems to suggest that dendritic spines are a crucial aspect in learning and memory. Their role in these crucial functions has been reflected by their absence in various neuropsychiatric disorders – such as schizophrenia, certain learning deficits present in some individuals with ASD, and memory deficits present in Alzheimer’s disease. These deficits seem to occur during the early creation of neural networks in the brain at synapses. Further research understanding the development of these spines and the creation of different morphological forms can be crucial in determining how to potentially cure or treat these deficiencies present in neuropsychiatric and learning disorders.
References
- NHS Choices, NHS, www.nhs.uk/conditions/schizophrenia/causes/.
- Bourne, Jennifer N, and Kristen M Harris. “Balancing Structure and Function at Hippocampal Dendritic Spines.” Annual Review of Neuroscience, U.S. National Library of Medicine, 2008, www.ncbi.nlm.nih.gov/pmc/articles/PMC2561948/.
- “Dendritic Spines: Spectrum: Autism Research News.” Spectrum, www.spectrumnews.org/wiki/dendritic-spines/.
- Hofer, Sonja B., and Tobias Bonhoeffer. “Dendritic Spines: The Stuff That Memories Are Made Of?” Current Biology, vol. 20, no. 4, 2010, doi:10.1016/j.cub.2009.12.040.
- Holtmaat, Anthony J.G.D., et al. “Transient and Persistent Dendritic Spines in the Neocortex In Vivo.” Neuron, Cell Press, 19 Jan. 2005, www.sciencedirect.com/science/article/pii/S0896627305000048.
- McCann, Ruth F, and David A Ross. “A Fragile Balance: Dendritic Spines, Learning, and Memory.” Biological Psychiatry, U.S. National Library of Medicine, 15 July 2017, www.ncbi.nlm.nih.gov/pmc/articles/PMC5712843/.
- Moyer, Caitlin E, et al. “Dendritic Spine Alterations in Schizophrenia.” Neuroscience Letters, U.S. National Library of Medicine, 5 Aug. 2015, www.ncbi.nlm.nih.gov/pmc/articles/PMC4454616/.
- Penzes, Peter, et al. “Dendritic Spine Pathology in Neuropsychiatric Disorders.” Nature Neuroscience, U.S. National Library of Medicine, Mar. 2011, www.ncbi.nlm.nih.gov/pmc/articles/PMC3530413/.
- “Schizophrenia.” Mayo Clinic, Mayo Foundation for Medical Education and Research, 7 Jan. 2020, www.mayoclinic.org/diseases-conditions/schizophrenia/symptoms-causes/syc-20354443.
- “Schizophrenia and Dendritic Spines.” Ness Labs, 20 June 2019, nesslabs.com/schizophrenia-dendritic-spines.
- “Synaptic Cleft: Anatomy, Structure, Diseases & Functions.” The Human Memory, 17 Oct. 2019, human-memory.net/synaptic-cleft/.
- Tian, Li, et al. “Activation of NMDA Receptors Promotes Dendritic Spine Development through MMP-Mediated ICAM-5 Cleavage.” The Journal of Cell Biology, Rockefeller University Press|1, 13 Aug. 2007, www.ncbi.nlm.nih.gov/pmc/articles/PMC2064474/.
- Zito, Karen, and Venkatesh N. Murthy. “Dendritic Spines.” Current Biology, vol. 12, no. 1, 2002, doi:10.1016/s0960-9822(01)00636-4.