By Carly Adamson, Neurobiology, Physiology & Behavior ‘21
Author’s Note: I wrote this literature review for a UWP 104E assignment for which we could pick any science topic that interested us. I chose neural crest cells (NCCs) because they are the research focus of Dr. Crystal Rogers’ developmental biology lab, which I intern for on campus, and have such diverse fates. When I wrote this piece, I had just started my internship, and I wanted to connect the lab’s research to my own interests in the peripheral nervous system. This review explains and connects five key discoveries within the history of NCC development research. My intention was to split my audience into both neurodevelopmental specialists and a broader group of biologists with little background in NCCs. I used the terminology necessary for this specialized analysis while also drawing main conclusions in simpler language. I relate to the reader with metaphorical and illustrative language, as I yearned for such explanations in my own exploration of this complex research topic.
Abstract
Schwann cell (SC) development through neural crest cell (NCC) migration and differentiation is a fascinating and important topic since these cells are critical for nervous system function. On the journey to becoming SCs, some Schwann cell precursors (SCPs) stay in their partially-differentiated state to guide other developing cells and to provide a ready supply of a variety of NCC derivatives whenever needed in development. There is a lot left to understand in this intricate process, including how the timeline of SCP development aligns with other neurodevelopmental processes. This research review focuses on key studies about the network of transcription factors, regulators, and enzymes that take multipotent cells from a central region to the final fate of SC maturity. This review also highlights Sox10, a key transcription factor, as a central point to ground the reader in all other discoveries surrounding SC differentiation.
Keywords: Schwann cells, Schwann cell precursors, Sox10, neural crest cells, neurodevelopment, glial growth factor
Introduction
Neural crest cells (NCCs) are the foundation for a variety of key structures, from pigment cells of the skin to neurons and glia of the periphery. NCCs are multipotent, meaning that they can continue to divide in an undifferentiated state as well as differentiate into a wide range of mature cell types. However, they are also the starting point for many pathologies in abnormal development, including digestive tract abnormalities, motor disabilities, and cancer [1]. These fascinating cells have long been shown to differentiate based on networks of environmental signals, as shown by extensive transplantation experiments [2]. All NCCs originate from a structure aptly named the neural crest (NC), which distinguishes vertebrates from other chordates [3]. There are many gene networks to pattern the differentiation, migration, and maintenance of pluripotency of these cells. NCCs must delaminate from the neural tube and migrate to their target tissues after a process known as epithelial-mesenchymal transition (EMT) to become diverse derivatives such as craniofacial bone, pigment, or neurons in the developing organism. Some NCCs even stay multipotent after migration to establish local stem cell populations [4]. NCCs were first described by Dr. Wilhelm His over 150 years ago, and so much about the cells’ diverse functions has been uncovered since then.
One of these diverse functions is the formation of Schwann cell precursors (SCPs). These NCC derivatives migrate along embryonic nerve fibers, supplying stem cells wherever needed in the embryo [5]. After a whirlwind of developmental signaling pathways and context-dependent regulation, mature Schwann cells (SCs) are created. SCs are a key player in the peripheral nervous system, providing insulation and structural support to nerves [6]. SCs create multilayered fatty structures called myelin sheaths that allow action potentials to quickly conduct along a nerve fiber. While a developing embryo must regulate many cell proliferation and differentiation processes at once, it is important to specifically balance SC myelination and differentiation to efficiently develop peripheral nerves that can relay information to their neighbors and listen to external signals. A failure to balance these processes can lead to motor and sensory disabilities in an individual. In addition, the number of SCs that proliferate must match the number of axons in a one-to-one relationship to properly sort cells for subsequent myelination [1]. This balance requires a hefty molecular team, each with key roles in guiding SCs to maturity.
A main player in SC development is Sox10, a protein that induces NCCs to differentiate into components of the peripheral nervous system. Sox10 is a transcription factor, meaning that it binds a specific DNA sequence to regulate the expression of other genes. Sox10 has been linked to a multitude of developmental processes, including three activities relevant to this review: the formation of the neural crest itself, the formation of the peripheral nervous system, and the complete differentiation process of SCs. Without the expression of the Sox10 gene, no glial cells can form in vivo or in vitro [6]. Since Sox10 plays such key roles in multiple stages of vertebrate development, its expression must be tightly regulated. In order to understand the path from multipotent NCCs to fully-developed SCs that keep the organism alive, one must dive into the complicated web of Sox10 control.
Identification of potential SC development factors
The significance of early work in SC development studies can be sorted into two categories: 1) setting the foundation for NCC isolation techniques and 2) identifying genes for further inquiry into their potential roles in SC development. A publication by Buchstaller et al. in the Journal of Neuroscience describes the genetic methods of expressing fluorescent proteins in mice to identify and isolate NCCs and developing SCs. It is important to obtain pure populations of NCCs to study their development and differentiation, as a clean starting point gives the most accurate results once specific induction factors are applied. These broad methods, along with the genetic protocols of RNA amplification and in situ hybridization, allowed later researchers to study many different NCC lineages. A notable candidate selected from this study is Oct6, a transcription factor that will be further discussed below for its role in SC development [7]. The work of Buchstaller et al. provided the foundation for further investigations into the roles of individual transcription factors and signaling proteins in SC differentiation.
Neuregulin-1/ErbB signaling
A key discovery earlier in neural crest experimentation was a particular environmental factor, glial growth factor (GGF), also known as neuregulin-1, that prevents rat NCCs from differentiating into neuronal cells and instructs them to instead differentiate into glial components, such as SCs. This environmental factor works to inhibit Mash1, a very early and essential marker for neuronal differentiation. By blocking Mash1, neuregulin-1 prohibits developing cells from ever starting down the path to neuronal maturity, suppressing the fate of neurons entirely. This result, along with rigorous experiments designed to replicate this finding, identified GGF as the first factor shown to both promote one NCC fate and suppress another [2]. This study changed the theory of SC differentiation by confirming neuregulin-1 as a key SC regulator and proposing the mechanism behind it.
Later research further investigated the role of neuregulin-1 in SC development by focusing on the protein’s interplay with Sox10. A 2020 study from Yang et al. found that the signaling pathway involving neuregulin-1 and its family of epidermal growth factor receptors, ErbB type, maintains expression of Sox10 in differentiating SCPs [6]. By uncoupling ErbB signaling from SC differentiation via two experimental groups, this study was able to show that the combination of ErbB2 and neuregulin are required to produce SC phenotypes and that neuregulin works by affecting Sox10 expression.
Oct6’s synergy with Sox10
Oct6 is a transcription factor that works synergistically with Sox10 to promote the myelination of SCs [6]. Jagalur et al. used cell culture and cloning methods in rat models to elucidate the role of Oct6 in connecting the regulation networks of the promyelinating SC, which has been paired with a neuron but lacks a complete myelin sheath, to the SC that actively ensheaths axons. The 2011 paper concluded through comparative genome studies that Sox10 proteins pair up to form structures called dimers and bind the Oct6 gene. This interaction creates a greater regulatory complex and allows developing SC populations to respond to environmental cues [8]. This study defines a key regulatory mechanism for timing the onset of SC myelination, which is highly important to neurodevelopment in an individual and acutely affects their prognosis. This understanding of Oct6 provides another piece to the puzzle of SC development: differentiating NCCs must be able to understand cues from pre-existing, mature cells.
Histone deacetylases modify multiple SC factors
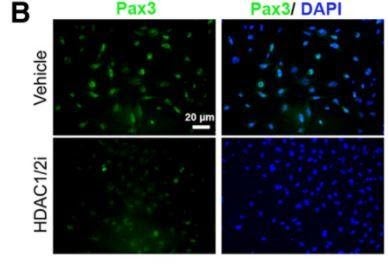
Figure 1: Bottom left image shows that the inhibition of HDAC1/2 yields lower Pax3 expression in JoMa1 cells. Image credit: Jacob et al., doi:10.1523/jneurosci.5212-13.2014.
Histone deacetylases are transcriptional regulators that remove acetyl groups from DNA histones to condense chromatin and to decrease DNA interactions with transcription factors. These enzymes can also remove acetyl groups from transcription factors themselves to modulate their activity. These enzymatic activities tie into SC development as demonstrated by Jacob et al.’s discovery in 2014 on the functions of histone deacetylase 1 and 2 (HDAC1/2). This study explains that HDAC1/2 are necessary for the myelination and complete maturation of SCs. These enzymes can be placed into the web of protein interactions that together regulate SC differentiation and myelination. This study used a combination of mouse neural crest explants and colonies, which differentiate into glial components in the presence of neuregulin, from the NCC-derived lineage called JoMa1 to get a more complete picture of transcriptional regulation. Jacob et al. show that HDAC1/2 unwind the tightly-packed DNA of the Pax3 gene to facilitate the expression of Pax3, an important transcription factor that maintains Sox10 [9]. This study produced a major change in theory due to the recognition of HDAC1/2 as induction factors for peripheral glia, including SCs, through their control of lineage-specific transcription factors, like Sox10.
Hippo/YAP/TAZ signaling
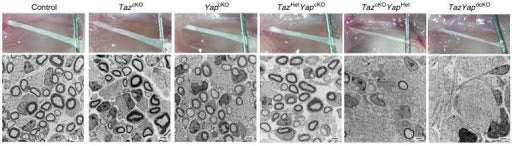
Figure 2: Control numbers and myelination of Schwann cells contrasts sharply with TAZ/YAP double knockouts (dcKO) on the far right. Image credit: Deng et al., doi: 10.1038/ncomms15161.
Hippo signaling refers to a pathway that affects cell proliferation and a process of controlled cell death known as apoptosis.It is primarily moderated by three factors: Hippo, TAZ, and YAP. TAZ and YAP proteins activate cell cycle regulators to promote proliferation of SC. The two also work with Sox10 to direct differentiation regulators for myelination [3]. TAZ and YAP were found to regulate SC proliferation via the control of cell cycle regulators and regulate SC myelination through interactions with Sox10. The direct targets of these two proteins are still not fully understood, but immunolabeling results published from Deng et al. in 2017 revealed TAZ and YAP to be necessary for SC proliferation and myelin induction. Single knockouts , which disable the target gene from functioning in an organism, for TAZ or YAP in mice showed how these two proteins can compensate for one another’s expression to produce normal SC phenotypes if only one factor is present. In contrast, double knockout mice showed a dramatic reduction in mature SCs due to decreased Sox10 expression [1]. The research that led to understanding the interplay of TAZ and YAP in this signaling network is key to SC developmental theory because the prognosis of mice without these genes is so bleak. Individuals without the proper number of mature SCs are not viable for long after birth due to severe motor and sensory deficits. These unfortunate phenotypes show the importance of TAZ and YAP in SC development as well as the importance of SCs to vertebrate life.
Further neuregulin-1/ErbB signaling analysis
Research on the neuregulin-1/ErbB signaling mechanism is key to understanding SC differentiation because this signaling makes important decisions early in differentiation that completely change the fate of a young NCC.
A closer look at Shah et al.’s 1994 Cell publication reveals advanced techniques that set an impressive foundation for future NCC studies. In order to understand neuregulin’s role in SC development, the paper aimed to answer the following question: When do NC-derived cells first start responding to neuregulins? Scientists used antibody staining, or immunocytochemistry, to fluorescently label NCCs with key proteins to track SC development as well as neuronal development for contrast. They stained for three proteins: 1) glial fibrillary acidic protein (GFAP) to tag immature SCs, 2) a mature Schwann cell-associated tyrosine kinase, c-Neu, and 3) peripherin to track cells developing into neurons. By analyzing NCC colonies from their early, undifferentiated state, scientists were able to study neuregulin’s instructional role in SC development.
The study found colonies of NCCs grown in neuregulin-1 to have no peripherin staining but intense GFAP staining, indicative of high levels of developing SCs and no developing neurons. This result suggests that the presence of neuregulin guides NCCs towards SC development and away from neuronal development. To confirm this result, scientists tested neuregulin-positive cell colonies for two additional Schwann cell-specific markers: P0 and O4. The presence of these protein markers in the neuregulin-treated colonies precisely identifies the cells as SCs, thus confirming neuregulin’s role in exclusively instructing SC differentiation. To confirm neuregulin’s unique role in promoting SC differentiation while suppressing neuronal differentiation, Shah et al. conducted careful colony analysis to confirm that the control and neuregulin-treated colonies had similar percentages of colony survival. This result shows that neurons were inhibited from ever forming in the first place rather than later degrading in neuregulin-positive media [2]. This experiment used rigorous colony analysis and previously validated protein markers to confirm their hypothesis of neuregulin’s two-pronged effects in directing NCC fate.
Yang et al.’s 2020 publication work builds upon Shah’s 1994 publication to examine the interplay between neuregulin and Sox10. This research is important because the study of individual protein factors is not enough to understand the complex control of a developmental process such as SC differentiation. The scientists isolated three groups of bone marrow mesenchymal stem cells (BMSCs) from mice: a control cell group with standard induction factors, a group in which neuregulin’s main receptor was blocked, and one group that never received any neuregulin. RT-PCR was used to quantify the amount of key transcriptional regulator proteins as a measure of the degree of stem cell differentiation into Schwann-like cells. The most significant results come from the ErbB2-inhibited cells treated with immunofluorescence staining, which exhibited significant decreases in multiple SC markers, large reductions in SC proliferation, and a significant decrease in Sox10 expression. These results show that ErbB2 and neuregulin are required to work together to induce stem cell differentiation into SCs. Further analysis of these results uncovered a positive feedback loop between neuregulin and Sox10, meaning the two factors’ reaction yields high amplification of the signals required to quickly create mature SCs in the developing embryo [6].
Conclusion
Current state of theory
As of this year, the entire path from NCCs to SCs is still not completely understood. It is difficult to create conditions that allow both NCC induction and the maintenance of their undifferentiated state. A second difficulty arises because new proteins are frequently added to a puzzle of cell signaling that is also not yet fully understood. There are only a few transcriptional regulators of NCCs that have been studied in detail, and little is known about the products of effector genes for the migration of NCCs [3]. As established in this review, at least four major cell signaling pathways are described to affect SC development. However, a comprehensive map of how these different pathways communicate and combine to deliver the product of mature SCs is not yet defined. Sox10 continues to be a key factor in SC differentiation, and its regulation proves to be more and more complicated with each new protein factor discovery. Nonetheless, the collective of rigorous science allows clarity to be found bit by bit, and there is great potential in the future of NCC-based therapeutics.
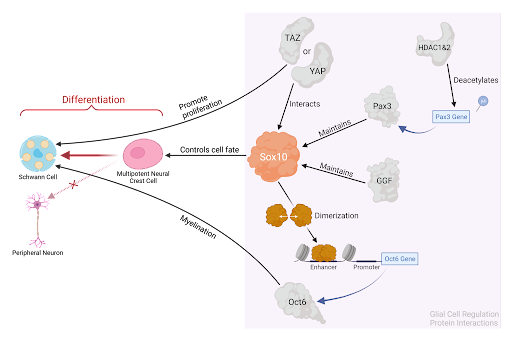
Figure 3: Summary of the molecular interactions between Sox10 and the four protein factors described in this review, guiding NCCs through the process of SC differentiation.
Important questions for future research
A key area of inquiry examines how this research on animal models might translate to regenerative medicine and stem cell-based therapeutics in humans. To be therapeutic for regenerative medicine, induced NCCs need to have as close to normal differentiation and population patterns as possible. This involves future research on functional comparisons between NC-derived stem cells in postnatal organisms and embryonic stem cells [5]. SCs hold a high potential for regenerative medicine due to their natural role in axonal regrowth following peripheral nerve damage [10]. The applications of this research are exciting, but there is still a long way to go in understanding the wide range of applicable protein activities.
Understanding normal patterns of SC development will help develop treatments for abnormal patterns, like SC tumors. Uncontrollable SC differentiation is a known characteristic of some cancers [1]. Recent research has identified a tumor suppressor, Nf2, that leads to Schwannomas and hyperplasia in mouse models when inactivated [3]. In addition, future manipulation of the Hippo signaling described in this article could compensate for myelin insufficiency without risking an overproduction of myelin that may lead to tumors [1]. The wide range of proteins described thus far as regulators ofSox10’s activity demonstrates the importance of continued funding for basic SC research. Finally, the modular content of this review supports the importance of further studies that focus on the interplay between cell signaling pathways to one day obtain a highly detailed, web-like recipe for SC differentiation.
References:
- Deng, Y., Wu, L. M. N., Bai, S., Zhao, C., Wang, H., Wang, J., et al. 2017. “A reciprocal regulatory loop between TAZ/YAP and G-protein Gas regulates Schwann cell proliferation and myelination.” Nat. Commun. 8, 1–15. doi: 10.1038/ncomms15161.
- Shah, N. M., Marchionni, M. A., Isaacs, I., Stroobant, P., & Anderson, D. J. 1994. “Glial Growth Factor Restricts Mammalian Neural Crest Stem Cells to a Glial Fate.” Cell, 77, 349-360.
- Méndez-Maldonado, K., Vega-López, G. A., Aybar, M. J., & Velasco, I. 2020. “Neurogenesis From Neural Crest Cells: Molecular Mechanisms in the Formation of Cranial Nerves and Ganglia.” Frontiers in Cell and Developmental Biology, 8: 1-15. doi:10.3389/fcell.2020.00635.
- Kunisada, T., Tezulka, K., Aoki, H., & Motohashi, T. 2014. “The stemness of neural crest cells and their derivatives.” Birth Defects Research Part C: Embryo Today: Reviews, 102(3), 251-262. doi:10.1002/bdrc.21079
- Perera, S. N., & Kerosuo, L. 2020. “On the road again – establishment and maintenance of stemness in the neural crest from embryo to adulthood.” Stem Cells Journals. doi:https://doi.org/10.1002/stem.3283
- Yang, X., Ji, C., Liu, X., Zheng, C., Zhang, Y., Shen, R., & Zhou, Z. 2020. “The significance of the neuregulin-1/ErbB signaling pathway and its effect on Sox10 expression in the development of terminally differentiated Schwann cells in vitro.” International Journal of Neuroscience, 1-10. doi:10.1080/00207454.2020.1806266
- Buchstaller J, Sommer L, Bodmer M, et al. 2004. “Efficient isolation and gene expression profiling of small numbers of neural crest stem cells and developing Schwann cells.” Journal of Neuroscience. 24: 2357-2365.
- Jagalur NB, Ghazvini M, Mandemakers W, et al. 2011. “Functional dissection of the Oct6 Schwann cell enhancer reveals an essential role for dimeric Sox10 binding.” Journal of Neuroscience.;31(23):8585–8594.
- Jacob, C., Lotscher, P., Engler, S., Baggiolini, A., Tavares, S. V., Brugger, V., Suter, U. 2014. “HDAC1 and HDAC2 Control the Specification of Neural Crest Cells into Peripheral Glia.” Journal of Neuroscience, 34(17), 6112-6122. doi:10.1523/jneurosci.5212-13.2014.
- Nishio, Y., Nishihira, J., Ishibashi, T., Kato, H., & Minami, A. 2002. “Role of Macrophage Migration Inhibitory Factor (MIF) in Peripheral Nerve Regeneration: Anti-MIF Antibody Induces Delay of Nerve Regeneration and the Apoptosis of Schwann Cells.” Molecular Medicine, 8(9), 509-520. doi:10.1007/bf03402160.