By Palak Arora
Author’s Note: I wrote this review article because it was an assignment for me, for the course UWP102B. We were instructed to choose any topic from the field of biology which presented me with a wide range of possibilities. I was not sure where to begin my search but one day while I was scrolling through social media, I heard about CAR T-cell therapy as a potential cure for cancer and I was very intrigued. The scientific community has been searching for this for a very long time and this new treatment is a huge breakthrough. This article will provide some background and explore research that has been done for CAR T-cell therapy. I want to bring awareness to my readers about this topic and also inspire young scientists to pursue research in this field since there are still many questions that have been left unanswered.
Abstract
The purpose of this review is to present the readers with an overview of the advancements in CAR T-cell therapy and areas in which more research is needed. CAR T-cell therapy is a modern approach in treating acute lymphoblastic leukemia. The modified T cells target a specific antigen on malignancies and help eliminate them. CD19 and CD22 are the antigens that are currently under investigation by researchers and the goal is to increase remission rates with the least amount of adverse events during recovery and prevent relapse as much as possible. Bispecific targeting of antigens and the subsequent use of allogeneic hematopoietic stem cell transplantation post-treatment are being examined as potential solutions to these challenges however further research is required to confirm these hypotheses and discover new approaches.
Introduction
Acute Lymphoblastic Leukemia (ALL) is the most common type of cancer in pediatric patients. This disease affects the blood and the bone marrow such that immature white blood cells are rapidly created from the bone marrow due to a genetic mutation that tells them to keep dividing. Lymphocytes are a type of white blood cells and there are two main types: B cells and T cells. ALL can affect both cell types but primarily affects B cells. In normal cases, mature B lymphocytes growing in the bone marrow function in the immune system by producing antibodies. But these leukemic cells are not as good at fighting infections, and as their numbers grow, they take up space that healthy red white blood cells could otherwise take [1]. Each B lymphocyte makes one kind of antibody that is highly specific for one unique antigen. These populations of B-cells are usually inactive, but as soon as one encounters a“non-self” or foreign antigen, the B cell is activated and begins to rapidly expand, producing multiple copies of itself. They display the antibody on their surface and as soon as they bind another antigen, they will also stimulate helper T cells which mount a defense against the “non-self” protein (an antigen). But the leukemic (cancerous) cells are not able to function properly due to an incomplete maturation process and are not as effective in fighting infections [1]. B-cell ALL is generally treated with chemotherapy or targeted immune cell therapy. After initial treatments, approximately 20% of pediatric and young adult patients relapse, suggesting chemotherapy alone is not enough to treat them [2]. Immunotherapy approaches that redirect T-cells to malignancies have been used in conjunction with chemotherapy, and have been proved to be effective in achieving complete remission (CR) [2]. These approaches include the use of tisagenlecleucel or CAR-T cell therapy, and Blinatumomab, a Bispecific T-cell engager (BiTE) which is a protein that simultaneously activates CD3 on T-cells and an antigen on the malignant cell in order to redirect T-cells towards the malignancy.
This review will focus on research from the years 2018-2022 in order to inform the readers about the current research in the field of CAR T-cell therapy to treat refractory or relapsed B-cell ALL in pediatric and adult patients. CD19 targeted CAR T- cell therapy has been successful in achieving high remission rates in pediatric patients but treating adult patients with B-cell ALL has been a significant challenge due to antigen loss post-infusion leading to a higher proportion of relapses and adverse events. Determining and preventing the risk factors for potential relapse is currently an active area of research.
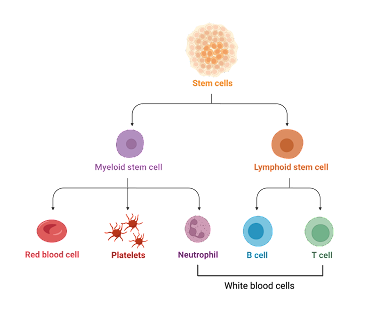
Figure 1. Stem cell differentiation: This disease affects the blood and the bone marrow such that immature white blood cells are rapidly created from the bone marrow due to a genetic mutation that tells them to keep dividing. Lymphocytes are a type of white blood cells and there are two main types: B cells and T cells. ALL can affect both cell types but primarily affects B cells.
What is CAR T- Cell Therapy?
Chimeric Antigen Receptor (CAR) T-cell therapy is a form of immunotherapy in which a patient’s white blood cells are modified and an artificial receptor, CAR, is added to it, allowing for recognition of specific antigens on cancer cells [3]. Through the process of leukapheresis, white blood cells are extracted and separated. These cells are used to generally target the CD19 antigen and are utilized by the FDA-approved medication tisagenlecleucel-T. After modifications, the cells are added back to the patient’s bloodstream and progress is measured by estimating complete remission rates and through biomarkers like minimal residual disease. Minimal residual disease is a term for the small number of cancer cells left in the body post-treatment.
Tisagenlecleucel vs Blinatumomab
Blinatumomab is another FDA-approved medication that is used to treat B-cell ALL. The major difference between the two approaches taken by Tisagenlecleucel vs Blinatumomab is that CAR-T cell therapy uses the 4-1BB co-stimulatory domain which enhances CAR-T cell proliferation while Blinatumomab does not. Verneris et al. (2021) conducted an experiment to indirectly compare the two medications and observed higher CR rates with tisagenlecleucel (82%) than with blinatumomab (39%). They also observed consistent higher overall survival (OS) rates in patients treated with tisagenlecleucel than those treated with blinatumomab. This study by Verneris et al. (2021) was informed by two major studies: ELIANA and MT103-205. The researchers utilized patient data from these previous studies to determine which immunotherapy approach, Blinatumomab or Tisagenlecleucel, is more safe and effective in treating acute lymphoblastic leukemia in pediatric and young adult patients. The previous studies were single-arm, so no comparisons could be made between the two types of treatment. However, Verneris et al. (2021) controlled for patient variables and used statistical analysis to make an indirect comparison between the two. They observed higher CR rates with tisagenlecleucel (82%) than with blinatumomab (39%). They also observed consistent higher OS rates in patients treated with tisagenlecleucel than those treated with blinatumomab. A potential third variable problem that could also explain these results is that patients in the ELIANA trial were heavily pre-treated before tisagenlecleucel infusion while those in the MT103-205 trial were not. Moreover, the ELIANA trial included pediatric and young adult patients while MT103-205 only included pediatric patients. This poses a significant difference in median ages, possibly affecting the results. The sample size in this study was large enough to be generalizable and the results proved to be statistically significant with a p-value of <0.0001for CR rates and a p-value of <0.001 for OS rates. This study is of relevance to newly enrolled patients with B-cell ALL who are considering their treatment options and might benefit by being able to compare these two types of treatments even if it was an indirect comparison [2]. While this indirect comparison provides a good starting point, further double-arm studies are needed to confirm these results.
Current challenges in CAR T- cell therapy
Dosage and Side effects
For CAR T-cell therapy to be effective, a minimum number of cells (108 CAR T-cells) need to be infused. Higher number of cells increases the chances of achieving remission. But increasing the number of cells also increases the side effects experienced by patients. One of the most common side effects experienced by 80% of patients is Cytokine Release Syndrome (CRS) which is a life-threatening consequence characterized by the dysfunction of multiple organs. Other side effects include febrile neutropenia characterized by a high risk of infection (experienced by 40% subjects), unresolved hematopoietic cytopenia by day 28 (characterized by a reduction in mature blood cells), transient neuro-psychiatric events, and tumor lysis syndrome (a large amount of tumor cells simultaneously releasing their contents into the bloodstream) [4]. Balancing the dosage of CAR T-cells without knowing which patients are at a higher risk of developing these side effects remains a significant challenge and is a potential area for research.
Antigen loss
CAR T-cell therapy functions by targeting antigens on malignant cells using artificial receptors. Researchers have observed, however, that after the initial infusion, more than 60% of these patients relapse due to CD19 antigen loss [5]. Without the antigen on the leukemic B-cells, the modified T cells are not able to bind to and eliminate them. This also means that a second infusion of CAR T-cells would not be helpful since CAR T-cell persistence is not the problem. It is not yet known whether antigen loss can be prevented but researchers are still looking into biomarkers for relapse.
Predicting and preventing relapse
Minimal residual disease (MRD)
Observing minimal residual disease is the most common way to determine the safety of CAR T-cell therapy in a clinical setting. There are two ways to measure this: Next-generation sequencing (NGS) and Flow cytometry. The NGS assay sequences and tracks rearranged tumor-specific immunoglobulin sequences while flow cytometry utilizes blood and bone marrow samples to measure the physical and chemical characteristics of individual cells to estimate the number of malignant cells. MRD positivity has been hypothesized to be a predictor of relapse and in their study, Pulsipher et al. (2022) present statistically significant results to affirm the hypothesis. The researchers also compared the two methods, NGS assay, and Flow cytometry, and learned that the Next Generation Sequencing technique was much more sensitive than Flow cytometry in detecting MRD positivity. After looking at multiple potential biomarkers and demographic characteristics, the researchers found no significant effect of age, cytogenic or genetic risk, sex, or prior therapy on relapse within a year after tisagenlecleucel infusion. They did however observe that persistence of B-Cell aplasia (CAR T-cells damaging normal B-cells) and detection of NGS-MRD to be high-risk factors for relapse [6]. Current research has been focusing on methods like allogeneic hematopoietic stem cell transplantation (allo-HSCT) to reduce MRD positivity and therefore prevent early relapse. Researchers are also looking into other possible antigens that might help reduce relapse rates.
Targeted Antigens
CAR-T cell therapy is designed to target specific antigens on malignant cells. The most common target is the CD19 antigen whose expression is maintained in most B-cell malignancies. According to the researchers, CD19 CAR-T cell therapy has proven to be a usually successful treatment option with a 70-90% complete remission rate for patients with relapsed or refractory B-cell ALL and yet a large proportion of patients have relapsed within a year [5]. The study by Maude et al. (2018) found that the overall remission rate was 82% in their sample of 50 patients using CD19 CAR T-cell therapy. The researchers used polymerase chain reaction (PCR) to detect Tisagenlecleucel in the peripheral blood and found no relationship between dosage and Tisagenlecleucel expansion. In their analysis of the safety of Tisagenlecleucel, they found that at least one adverse event occurred in all patients. These adverse events included, but are not limited to, cytokine release syndrome, pyrexia, decreased appetite, febrile neutropenia, and headache. Most importantly, 19 patients died after Tisagenlecleucel infusion. The study concludes that tisagenlecleucel produces high remission rates in high-risk pediatric and young adults with relapsed or refractory B-cell ALL although there are significant risks associated with this approach [7].
Therefore an alternative antigen CD22 is also under investigation as a potential solution. Shah et al. (2020) investigated the lack of alternative immunotherapy treatment options for people with B-cell ALL who have relapsed after CD19 CAR-T cell therapy. They learned that 86.2% of the participants developed Cytokine Release Syndrome (CRS), Hemophagocytic Lymphohistiocytosis (HLH) like toxicities only developed in patients who had CRS, and peak
CAR expansion occurred 14-21 days after infusion. The researchers observed that 70.2% of the participants achieved complete remission, and 87.5% of these were found to be negative for Minimal residual disease (MRD) by Flow cytometry. And 75% of the participants experienced a relapse. They conclude that CD22 CAR-T cell therapy is a highly effective treatment option for those who have experienced relapse after CD19 CAR-T cell therapy or for those who were resistant to it [8]. Moreover, the study by Pan et al. (2019) has also shown promising results with a 70.5% complete remission rate among patients with refractory or relapsed B-cell ALL [5]. Targeting these antigens by themselves has achieved high CR rates and yet relapse continues to be an issue.
Another alternative is to target the CD19 and CD22 antigens simultaneously. In this approach, researchers can edit the patient’s T cells in a way that it has a receptor for both the CD19 and CD22 antigens. This technique is used to overcome antigen loss—If one of the antigens is lost, another is still available for T-cells to target, therefore improving response rates to therapy. After reviewing the results of their experiments Dai et al. (2020) conclude that these bispecific CD19/22 CAR-T cells might provide a good alternative for adult patients with B-cell ALL who are ineligible for other treatments as this immunotherapy option is able to prevent antigen escape without an increased risk of toxicity [9].
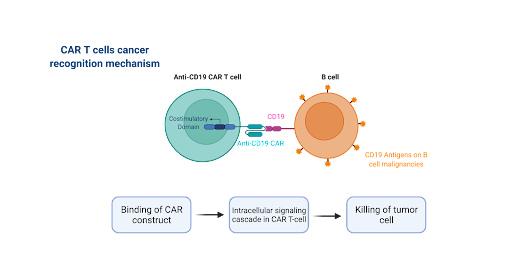
Figure 2. CAR T-cells are programmed to attack specific antigens like CD19 or CD22 on malignant cells.
Allogeneic hematopoietic stem cell transplantation (allo-HSCT)
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a procedure in which healthy stem cells (blood-forming cells) are transferred from a donor to a patient in order to replace the patient’s own stem cells. This procedure can be used post CAR T-cell therapy as a way to increase MRD negativity by increasing the number of healthy blood cells to improve patient recovery. In their study, Jiang et al. (2019) claim that despite the promising results of CAR-T cell therapy, patients are at a high risk of relapse due to antigen escape from tumor cells and reduced CAR-T cell persistence. They, therefore, decided to investigate whether subsequent allo-HSCT could increase minimal residual disease (MRD) negativity. This was a quantitative study that observed the number of patients with adverse effects post-treatment, the one-month remission rate (CR), overall survival (OS), event-free survival (EFS), relapse-free survival (RFS), and in vivo persistence of CAR-T cells. The researchers utilized real-time quantitative polymerase chain reaction (qPCR) to measure the level of CAR gene and flow cytometry to calculate the percentage of CAR-T cells in the peripheral blood and bone marrow post-infusion. Jiang et al. (2019) observed significant differences in the EFS and RFS rate between the two groups. However, no significant differences were observed in the overall survival rate [10].
Several studies have attempted to investigate the effects of allo-HSCT in combination with CAR-T cell therapy but so far, the results have been limited and on multiple occasions contradictory. Since this is an urgent problem that needs to be addressed, Jiang et al. (2019) designed their study in an attempt to replicate and confirm the benefits of allo-HSCT. While this study observed an improvement in EFS in their experimental group, there was a previous study that observed no such differences. Jiang et al. (2019) caution that while their study has been indicative of trends, due to the short-term follow-up and non-randomization, further studies need to be conducted on a larger scale to obtain more reliable and valid results [9].
Conclusion
In conclusion CAR T-cell therapy is an effective way to treat B-cell ALL and it has been able to achieve complete remission in up to 90% of patients in clinical trials. Achieving complete remission is relatively easier in pediatric patients but both pediatric and adult patients are still very likely to suffer from relapse within the same year due to antigen loss among other reasons. This field needs a lot of research to improve patient outcomes, achieve higher remission rates, and prevent relapse as much as possible. While researchers have been able to identify high risk factors for relapse such as B-cell aplasia and minimal residual disease positivity, it is not yet known how these can be effectively reduced. Allogeneic hematopoietic stem cell transplantation might be one potential way to prevent relapse but so far, the results are very limited, and they often contradict each other. Clinical trials with a larger sample size are needed in this area of research to provide accurate and reliable results. Overall, CAR T-cell therapy has proven to be one of the greatest advancements in cancer treatments in the past ten years but the treatment plan needs a lot more refinement before it can be used to its full potential.
References:
- National Cancer Institute. 2021 Nov. 19. Adult Acute Lymphoblastic Leukemia Treatment (PDQ®)–Patient Version. Accessed 2022 Feb 11. Available from: https://www.cancer.gov/types/leukemia/patient/adult-all-treatment-pdq
- Verneris MR, Ma Q, Zhang J, et al. 2021. Indirect comparison of tisagenlecleucel and blinatumomab in pediatric relapsed/refractory acute lymphoblastic leukemia. Blood Advances. 5(23):5387-5395. doi:10.1182/bloodadvances.2020004045
- American Cancer Society. CAR T-cell Therapy and Its Side Effects. Accessed 2022 Feb11. Available from: https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/immunothe rapy/car-t-cell1.html
- Thomas X, Paubelle E. 2018. Tisagenlecleucel-T for the treatment of acute lymphocytic leukemia. Expert Opinion on Biological Therapy. 18(11):1095-1106. doi:10.1080/14712598.2018.1533951
- Pan J, Niu Q, Deng B, et al. 2019. CD22 CAR T-cell therapy in refractory or relapsed B acute lymphoblastic leukemia. Leukemia 33(12): 2854-2866. doi:10.1038/s41375-019-0488-7
- Pulsipher MA, Han X, Maude SL, et al. 2022. Next-Generation Sequencing of Minimal Residual Disease for Predicting Relapse after Tisagenlecleucel in Children and Young Adults with Acute Lymphoblastic Leukemia. Blood Cancer Discovery 3(1):66-81. doi:10.1158/2643-3230.BCD-21-0095
- Maude SL, Laetsch TW, Buechner J, et al. et al. 2018. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. New England Journal of Medicine 378:439-448.
- Shah NN, Highfill SL, Shalabi H, et al. 2020. CD4/CD8 T-Cell Selection Affects Chimeric Antigen Receptor (CAR) T-Cell Potency and Toxicity: Updated Results From a Phase I Anti-CD22 CAR T-Cell Trial. JCO. 38(17):1938-1950. doi:10.1200/JCO.19.03279
- Dai H, Wu Z, Jia H, et al. 2020. Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia. Journal of Hematology & Oncology. 13(1):30. doi:10.1186/s13045-020-00856-8
- Jiang H, Li C, Yin P, et al. 2019. Anti-CD19 chimeric antigen receptor-modified T-cell therapy bridging to allogeneic hematopoietic stem cell transplantation for relapsed/refractory B-cell acute lymphoblastic leukemia: An open-label pragmatic clinical trial. American Journal of Hematology. 94(10):1113-1122. doi:10.1002/ajh.25582