By Clara Brewer, Neurobiology, Physiology, and Behavior ’22
Author’s Note:
In 2015, I was diagnosed with a rare pain disorder- Complex regional pain syndrome (CRPS). Not only does this disorder cause unimaginable pain, it is also virtually invisible to others, creating a discrepancy between the outside world’s perception of CRPS and the actual struggle that CRPS patients deal with, both physically and emotionally. Current trends for CRPS treatment are focused on the physical aspects of the disorder- increasing mobility and use of the affected limb. Oftentimes, this approach fails to treat the simultaneous psychological changes that can increase a patient’s risk for developing a concurrent mental health disorder. Even though I had access to a top CRPS treatment facility, I still experienced depression and anxiety which made my recovery from CRPS much harder. While writing a different paper focused on educating newly diagnosed individuals on the causes, symptoms, and available treatment options for CRPS, I realized that most of my findings addressed the physical symptoms and not the psychological changes. This discrepancy between both my own experiences and the experiences of many others who were diagnosed with CRPS and the treatment options available inspired me to try to understand this connection between CRPS and the increased diagnosis of comorbid mental health disorders.
While many students who read this paper may not go on to change the treatment for one rare disorder- it is important for anyone who wants to go into the medical field to begin reshaping their approach to medicine by reading articles like mine. I hope to shed light on the importance of reevaluating current treatment protocols for a wide range of disorders to include more mental health support for patients- a topic directed for students hoping to pursue a career in the medical field. By viewing medical diagnosis- in this case CRPS- as an interconnection between mind and body, future medical professionals will be able to holistically address disorder, instead of treating only the more obvious physical symptoms.
Complex regional pain syndrome (CRPS), a neuroinflammatory disease, ranks number one on the McGill Pain Scale, topping fibromyalgia, cancer, and amputation without anesthetics. The development of CRPS typically occurs after trauma to the arm or leg (e.g., breaking of a bone, dislocation of a joint, surgical trauma to a limb) that results in a disproportionately high sensation of pain. This painful response is chronic and characterized by constant pain with additional flare-ups that last different amounts of time from person to person. As a result, those with CRPS will often experience perpetual pain that can be made even worse with stress.
After the initial injury and subsequent chronic pain, a CRPS diagnosis follows the Budapest Diagnostic Criteria, where patients must report symptoms in three of four categories and must present symptoms in two of four categories at the time of evaluation. The categories are as follows: sensory, vasomotor (relating to blood vessels), sudomotor (relating to sweat glands), and motor/tropic (relating to muscles and bones). Symptoms include swelling of the limbs, skin discoloration, abnormal sweat response, and painful responses to non-painful or slightly painful stimuli, among others [1].
CRPS affects 200,000 people in the United States each year. Among those affected, half are also diagnosed with a psychiatric disorder [2]. More specifically, CRPS patients have a much higher prevalence of depression than the general population, with 15.6 percent of CRPS patients diagnosed with depression compared to 3.4 percent of people diagnosed with depression worldwide [2]. Since the pain in CRPS is so intense and the length of a painful flare-up varies from person to person, many patients come to develop a fear of the pain itself, altering their fear-brain circuits and creating a negative relationship between pain and their psychological state [3]. Mood disorders like depression and anxiety also have similar pathophysiology as CRPS, so the onset of CRPS can instigate the development of a comorbid mood disorder without an external trigger like grief, loss, or substance abuse. Within the physiology of CRPS, cytokine and astrocyte levels become dysregulated, mimicking the pathophysiology noted in certain psychiatric disorders and thus increasing rates of comorbid psychiatric disorders. Similarly, the fear-brain circuit is altered during the onset and prolonged management of CRPS, eventually transforming from pain-related fear to an overall manifestation of anxiety.
While the number of patients being diagnosed with comorbid mood disorders is growing, the current CRPS treatment protocol does not typically include the management of these psychiatric disorders. As more research is conducted to explore the physiological and psychological changes that occur with the onset of CRPS, mounting evidence suggests more mental health resources should be provided to alleviate CRPS-related symptoms, both physiological and psychological, and speed up the recovery timeline [4].
Dysregulation of Biomarkers in CRPS and Mood Disorders
TNF-α and Cortisol
During CRPS, depression, and anxiety, plasma levels of the pro-inflammatory cytokine tumor necrosis factor-α (TNF-α) are increased by a statistically significant degree [5]. This cell-signaling protein is integral to maintaining a healthy immune response and stimulates the release of a corticotropin-releasing factor. With CRPS, TNF-α activates and sensitizes primary afferent nociceptors, leading to the characteristic excessive pain [5]. TNF-α not only plays an important role in the development of CRPS, but also in the development and severity of depression. In fact, the more severe the reported depressive symptoms, the higher the concentrations of plasma TNF-α [6].
The increased concentration of TNF-α may be explained by its role in the regulation of the hypothalamic pituitary adrenal (HPA) axis which is responsible for neuroendocrine modulation of the body’s stress response. With an upregulation of pro-inflammatory cytokines like TNF-α in CRPS, the HPA axis increases the secretion of a corticotropin-releasing factor, adrenal-corticotropin hormone, and eventually cortisol. Prolonged elevations of cortisol lead to a shift in tryptophan usage from the tryptophan-serotonin pathway to the tryptophan-kynurenine pathway instead [7]. The shift away from the tryptophan-serotonin pathway greatly limits the production of serotonin, eventually interfering with mood stabilization, sleep cycle regulation, and neuronal communication [8]. Metabolites are then used in the tryptophan-kynurenine pathway instead, leading to the production of two neurotoxic chemicals, 3-hydroxyanthranilic acid, and quinolinic acid.
Quinolinic acid contributes to neurodegeneration seen in conditions like depression through free radical formation, mitochondrial malfunction, and energy store depletion. These changes trigger the mass destruction of neuronal cells which leads to the degeneration of brain functions such as memory and learning [9]. In fact, it has been suggested that elevated TNF-α levels is one precursor to the development of depression [5]. Therefore, TNF-α could be a potential biomarker for comorbid depression in CRPS patients.
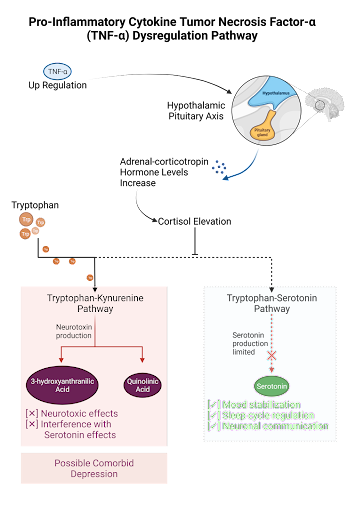
Figure 1: TNF-a’s role in the dysregulation of tryptophan metabolism. Under normal conditions, tryptophan is transformed into serotonin in the brain and gut, producing regulatory effects on mood and the sleep cycle as well as promoting health communication between neurons. Under inflammatory conditions, like those seen in CRPS and depression, TNF-a secretion inhibits the production of serotonin through the upregulation of adrenal-corticotropin. Tryptophan is then utilized in the liver for production of neurotoxins through the Kynurenine pathway.
Catecholamines
Following the acute stage of CRPS, stimulation of the sympathetic nervous system and the resulting release of catecholamines increase the production of another important cytokine, interleukin-6 (IL-6). When the body undergoes acute stress, the sympathetic nervous system is activated and causes the release of two catecholamines, epinephrine and norepinephrine. These hormones increase blood pressure, heart rate, breathing rate, and dilate the pupils. Catecholamines are also regulators of IL-6, a cytokine that plays an important role in nociceptor sensitivity while also increasing chances of developing comorbid anxiety. Norepinephrine upregulates the translation of IL-6 by 49-fold, therefore increasing the plasma concentration of IL-6 after sympathetic stimulation [10]. In the case of CRPS, the initial trauma to the affected limb activates this fight-or-flight response and increases IL-6 levels. The chronic elevation of IL-6 not only leads to chronic inflammation, but also increases nociceptor sensitization and the transmission of signals between sensory neurons, both of which are linked to chronically elevated levels of pain [11].
Not only does IL-6 play an important role in inflammatory and pain responses and the onset of CRPS, the cytokine also modulates the expression of another cytokine, interleukin-1 (IL-1). IL-1 is critical for the onset of anxiety-type symptoms by dampening the activation of endocannabinoid receptor CB1R (GABA), which limits GABA’s anti-anxiety effects [12]. In fact, general anxiety disorder patients had statistically significant high levels of IL-6 through environmental stimulation of the sympathetic nervous system [7]. By adding the excitatory effects of CRPS on the sympathetic nervous system, there is no need for external stimulation to begin anxiety symptoms. For this reason, it has been suggested that elevated levels of IL-6 from the onset of CRPS can stimulate IL-1 and induce comorbid anxiety [12].
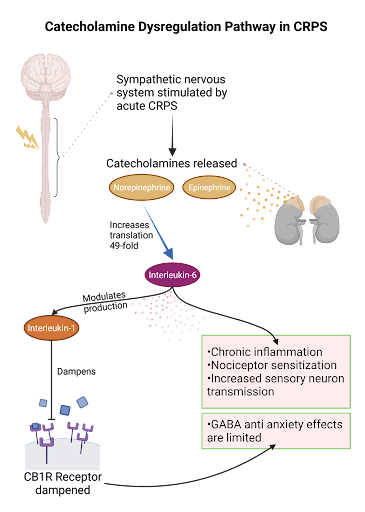
Figure 2: Sympathetic stimulation following acute CRPS results in an increase in catecholamines. The subsequent upregulation of interleukin-6 and interleukin-1 dampens GABA’s anti-anxiety effects and leads to increased nociceptor activation.
Astrocytes
In conjunction with the changes to cytokines in CRPS, anxiety and depression, subsequent changes to the functioning of astrocytes have been noted in all three diagnoses [13]. Astrocytes are a subclass of glial cells that hold a supportive function for neurons. Under typical conditions, astrocytes are responsible for modulating neuroendocrine functions, regulating synaptic transmission, and regulating glutamate levels in the body [14]. With CRPS, astrocytes become upregulated and activated via stimulation from excess pro-inflammatory cytokines, changing their gene expression to become A1 reactive astrocytes. A1 reactive astrocytes then go on to secrete neurotoxins and more pro-inflammatory cytokines [15]. The activation of A1 reactive astrocytes also induces higher levels of glial fibrillary acidic protein, thus increasing the number of glial glutamate transporters on astrocytes [16]. This hyperactivity and hypersensitivity of astrocytes to glutamate trigger calcium release that increases neurotransmission within nociceptors and ultimately contributes to the intense and chronic pain associated with CRPS [15].
Additionally, patients with major depressive disorder show an mean increase of 35 μmol/L of glutamate concentration in the cortex, a statistically significant changeincrease that is indicative of the severity of depressive symptoms [17]. The shift in gene expression in astrocytes and upregulation of glutamate with the onset of CRPS not only increases nociceptor activation but also neurodegeneration through the reinforcement of the tryptophan-kynurenine pathway discussed earlier. Interestingly, the increased concentration of glutamate inhibits the production of kynurenic acid and instead promotes the production of quinolinic acid [18]. As discussed, quinolinic acid is a potent neurotoxic compound that can lead to neurodegeneration associated with depression. The upregulation and activation of A1 reactive astrocytes in response to inflammation from CRPS increases extrasynaptic glutamate concentrations, causing hyperactivation of nociceptors and increased production of quinolinic acid. With this in mind, there is evidence that the pathophysiology of astrocytes during CRPS may increase the risk of comorbid depression.
CRPS Psychology: Cycle of Pain-Related Anxiety
The fear of pain, also known as harm-avoidance, complicates treating chronic pain conditions like CRPS. This is because certain treatments like physical or occupational therapy methods used in CRPS rehabilitation can quickly become unsuccessful if patients begin to avoid activities that increase symptoms or pain. These treatment techniques can include graded motor imagery, range-of-motion exercises, mirror therapy, desensitization, and electrical stimulation. Although these rehabilitation techniques may temporarily increase CRPS-related pain, therapy is an essential part of treatment and by avoiding painful activities, not only does the CRPS become increasingly worse, but the fear of pain is also cognitively reinforced.
This reinforcement is seen in the fear-learning neural pathway, a series of neurons that extend from the left amygdala to the hippocampus, cerebellum, brainstem, and other parts of the central nervous system. The chronic pain of CRPS and subsequent fear of pain continuously activates neurons that extend to this fear-learning circuit, strengthening the connection between the left amygdala, the fear control center, and the hippocampus, the learning and memory center. This repetitive activation ultimately intensifies the fear brain circuit [19]. Consequently, the cycle of pain-related anxiety begins, transitioning from a fear of pain to an avoidance of pain which then further reinforces the fear of pain [2]. This phenomenon is identified in multiple studies, suggesting a more quantitative link between CRPS and anxiety.
In one study, a group of 64 CRPS patients were evaluated to determine psychological comorbidities. Twenty-eight individuals received a psychiatric diagnosis following the onset of CRPS, with 10 of those 28 diagnosed with anxiety disorders [20]. Not only did the study reveal these diagnoses, they also found that increased anxiety was directly linked to increased pain through the fear-brain pathway [13]. In fact, another study reported that 70 percent of CRPS patients had elevated pain-related fear scores [19]. While the anticipation of pain can elicit pain response, it can also lead to an avoidance of daily responsibilities, physical inactivity, disability, poorer long-term recovery, and higher rates of anxiety and other mood disorders [20]. As a result, the altered fear-brain circuits associated with CRPS increase the likelihood of developing comorbid anxiety.
Conclusion
CRPS alters the body’s physiology and changes certain psychological processes, increasing the chances for developing a comorbid psychiatric disorder. With the dysregulation of cytokines and astrocytes, the immune system’s functioning is disturbed, which leads to abnormal levels of kynurenine and serotonin similar to that of depression [5]. As the levels of kynurenine increase, so does the concentration of extrasynaptic glutamate, upregulating processes that signal both pain and depression [9]. The fear-brain circuit is also altered as pain signals become stronger and more frequent with CRPS, catastrophizing pain and ultimately leading to elevated levels of both pain and anxiety [3]. While pain itself can be debilitating, the simultaneous occurrence of pain and comorbid psychiatric disorders seen in CRPS can lead to avoidance of daily life, thereby worsening both disorders.
Today, most patients managing CRPS disorders are reluctant to express their psychological symptoms and look for help on their own, yet neglecting the psychological side often worsens the symptoms of their diagnosis and makes recovery more difficult [19].Current research suggests that CRPS treatment should shift towards focusing on the psychological components that intensify pain in order to holistically treat CRPS. Over the past few years, more studies have explored the relationship between CRPS and psychiatric disorders, but there has been less research into treatments that would help with both disorders simultaneously. Just as CRPS is often misdiagnosed as an invisible disorder, psychological symptoms may be overlooked or undertreated when physiological responses garner priority. A new perspective of CRPS that acknowledges this association is needed to gain a more comprehensive understanding of the disorder.
References:
- Wie, C., Gupta, R., Maloney, J. et al. 2021. Interventional Modalities to Treat Complex Regional Pain Syndrome. Curr Pain Headache Rep. 25, doi:10.1007/s11916-020-00904-5
- Bean,. J., Johnson, H., Heiss-Dunlop, W., Lee, C., & Kydd, R. 2015. Do psychological factors influence recovery from complex regional pain syndrome type 1? A prospective study. Pain. 156(11), 2310–2318, doi:10.1097/j.pain.0000000000000282
- Antunovich, D.,, Horne, J., Tuck, N., Bean, D. 2021. Are Illness Perceptions Associated with Pain and Disability in Complex Regional Pain Syndrome? A Cross-Sectional Study. Pain Medicine. 22 (1): 100–111, doi:10.1093/pm/pnaa320
- Park HY, Jang YE, Oh S, Lee PB. 2020. Psychological Characteristics in Patients with Chronic Complex Regional Pain Syndrome: Comparisons with Patients with Major Depressive Disorder and Other Types of Chronic Pain. J Pain Res. 13:389-398, doi:10.2147/JPR.S230394
- Üçeyler, N., Eberle, T., Rolke, R., Birklein, F., Sommer, C. 2007. Differential expression patterns of cytokines in complex regional pain syndrome. Pain. 132(2007): 195-205. doi: 10.1016/j.pain.2007.07.031
- Zou, W., Feng, R., Yang, Y. 2018. Changes in the serum levels of inflammatory cytokines in antidepressant drug-naive patients with major depression. Plos One. 13(6): e0197267. doi: 10.1371/journal.pone.0197267
- Strong, J., Jeon, S., Jeon, S., Zhang, J., & Kim, Y. 2020. Glial Cells and Pro-inflammatory Cytokines as Shared Neurobiological Bases for Chronic Pain and Psychiatric Disorders. Overlapping Pain and Psychiatric Syndromes: Global Perspectives. doi:10.1093/med/9780190248253.003.0003
- Carhart-Harris, R. & Nutt, D. 2017. Serotonin and brain function: a tale of two receptors. J Psychopharmacol. 31(9): 1091-1120. doi: 10.1177/0269881117725915
- Pérez-De La Cruz, V., Carrillo-Mora, P., & Santamaría, A. 2012. Quinolinic Acid, an endogenous molecule combining excitotoxicity, oxidative stress and other toxic mechanisms. Int J Tryptophan Res. 5: 1–8, doi: 10.4137/IJTR.S8158
- Burger, A., Benicke, M., Deten, A., Zimmer, H. G. (2001). Catecholamines stimulate interleukin-6 synthesis in rate cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 281: H14-H21. doi:10.1152/ajpheart.2001.281.1.H14
- Zhou, YQ., Liu, Z., Liu, ZH., Chen, SP., Li, M., Shahveranov, A., Ye, DW., & Tian, YK. 2016. Interleukin-6: an emerging regulator of pathological pain. J Neuroinflammation. 13: 141. doi: 10.1186/s12974-016-0607-6
- Rossi, S., Sacchetti, L., Napolitano, F., De Chiara, V., Motta, C., Studer, V., Musella, A., Barbieri, F., Bari, M., Bernardi, G., Maccarrone, M., Usiello, A., Centonze, D. 2012. Interleukin-1β cauSeS anxiety by interacting with the endocannabinoid system. J Neurosci. 32(40): 13896-13905. doi: 0.1523/JNEUROSCI.1515-12.2012
- Ji, RR., Donnelly, C.R. & Nedergaard, M. 2019. Astrocytes in chronic pain and itch. Nat Rev Neurosci. 20: 667–685, doi:10.1038/s41583-019-0218-1
- Jauregui-Huerta, F., Ruvalcaba-Delgadillo, Y., Gonzalez-Castaneda, R., Garcia-Estrada, J., Gonzalez-Perez., Luquin, S. 2010. Response of glial cells to stress and glucocorticoids. Curr Immunol Rev. 6(3): 195-204. doi: 10.2174/157339510791823790
- Li, T., Chen, X., Zhang, C., Zhang, Y., & Yao, W. 2019. An update on reactive astrocytes in chronic pain. J Neuroinflammation. 16: 140. doi: 0.1186/s12974-019-1524-2
- Wesseldijk, F., Fekks, D., Huygen, F., van de Heide-Mulder, M., Zijlstra, F. 2008. Increased plasma glutamate, glycine, and arginine levels in complex regional pain syndrome type 1. Acta Anaesthesiol Scand. 52(5): 688-94. doi: 10.1111/j.1399-6576.2008.01638.x
- Mitani, H., Shirayama, Y., Yamada, T., Maeda, K., Ashby, C., Kawahara, R. 2006. Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Pro Neuro Psychoph. 30(6): 1155-1158. doi: 10.1016/j.pnpbp.2006.03.036
- Schwarcz, R. & Stone, T. 2018. The kynurenine pathway and the brain: challenges, controversies and promises. Neuropharmacology. 122(Pt B): 237-247. doi: 10.1016/j.neuropharm.2016.08.003
- Simons, L. E. 2016. Fear of pain in children and adolescents with neuropathic pain and complex regional pain syndrome. Pain. 157: 90-97, doi:10.1097/j.pain.0000000000000377
- Brinkers, M., Rumpelt, P., Lux, A., Kretzschmar, M., & Pfau, G. 2018. Psychiatric Disorders in Complex Regional Pain Syndrome (CRPS): The Role of the Consultation-Liaison Psychiatrist. Pain Res Manag. doi:10.1155/2018/289436

