By Elexia Butler, Human Biology, ’23
Author’s Note: This article was written to reveal how the COVID-19 vaccines are produced and how they are a safe technology used to help reduce the number of sick individuals. Throughout the article, I will discuss the safety and efficacy of mRNA vaccines as well as the limitations that scientists overcome. I chose this topic because mRNA vaccines are a “new” technology that many of us don’t understand and has led to a larger social debate. The controversy surrounding mRNA vaccines stems from people’s questions regarding the vaccines’ safety and necessity. After reading this article, I hope the reader is able to take away the fact that the mRNA vaccines are safe and effective.
Abstract:
As the world begins to settle after the past year and a half of operating with the COVID-19 pandemic, we look to mRNA vaccines to help return to a sense of normalcy. With both Moderna and Pfizer leading the market of mRNA vaccines since April 2021, we have seen a large decline in cases [27]. However, many people across the country are still skeptical of this “new” mRNA vaccine technology [8] and remain hesitant about getting the vaccine. Additionally, the COVID-19 vaccine controversy has left many individuals wondering if the vaccine is truly a safe way to fight the spread of COVID-19 or not. Currently, 54.7-59% of Americans have been fully vaccinated, but based on a PBS poll 24% have chosen to not receive any dose of the vaccine [40-41]. The goal of this article is to demonstrate the safety of mRNA vaccines, their development, limitations, and potential for treating future diseases.
Introduction:
Messenger RNA (mRNA) vaccines are not a new technology, in fact they have been researched for years. mRNA is a small genetic molecule that encodes specific proteins [33]. The discovery of mRNA in 1961 sparked an entire field of research related to gene regulation [1-5].
Traditional vaccines work by introducing an antigen (a foreign substance that is recognized by the immune system) to elicit an immune response and cause the body to produce antibodies against that antigen [13]. For nucleic acid vaccines (DNA and RNA vaccines), rather than directly injecting the antigen, the instructions for producing the antigen are introduced into the cell [14]. The cell can then use these instructions to “make a protein—or even just a piece of a protein—that triggers an immune response inside our bodies” [16]. In the case of COVID-19, Pfizer and Moderna mRNA vaccines encode the instructions to make a viral spike protein from SARS-COV-2 (the virus that causes COVID-19). The spike protein won’t cause sickness on its own, it trains the immune system to defend against the real SARS-COV-2 virus [38]. While research has been conducted on both DNA and RNA based nucleic acid vaccines, it has been shown that RNA vaccines are able to elicit a stronger immune response and are likely safer [15]. The technology of mRNA vaccines became increasingly promising as scientists used the speed of production of the technology to develop a safe and effective mRNA vaccine to their advantage [50-51]. One of the many reasons the Moderna and Pfizer vaccines work is the way they modify the stability of the mRNA and establish a method for efficient delivery, allowing for a strong immune response when administered [17, 45-47]. Though hesitancy remains surrounding the COVID-19 vaccine, the Moderna and Pfizer vaccines are both effective and have significantly reduced the infection rate of COVID-19 [27]. This hesitancy has been fueled by reports of conspiracies as well as possible health effects, which all have been proven false and will be discussed later in larger detail.
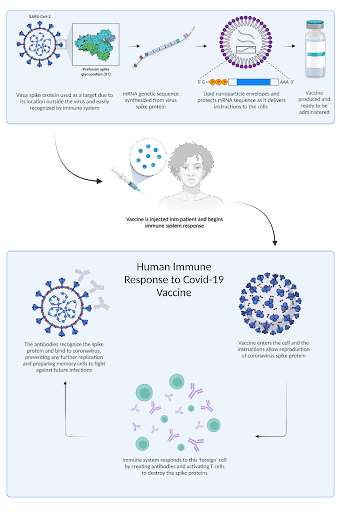
Figure 1. This diagram demonstrates how the SARS-COV-19 vaccine was produced and how it elicits an immune response. Through the mRNA being introduced into the body, the cells gain instructions on how to produce the spike protein and forms antibodies.
Proof of Principle:
The COVID-19 mRNA vaccine has brought hope to the medical field because they are effective and can continue to develop. With this technological advancement, it is important to maintain a certain standard of success to build confidence in the vaccines. The Food and Drug Administration (FDA) has set a standard for success of “at least 50%” efficacy, or the prevention of the spread of infection due to the vaccine [18, 53]. The Moderna and Pfizer mRNA vaccine clinical trials exceeded this standard, granting them Emergency Use Authorization (EUA). The application of the mRNA vaccine demonstrated an effectiveness of “90% for full immunization and 80% for partial immunization” [10]. A study, conducted by the CDC in March of 2021, was used to assess the real world application and effectiveness of the vaccine in a potentially infectious setting. As reported by the CDC, the group of vaccinated first responders and essential health care workers were prevented from infection. This study showed that the Moderna and Pfizer vaccines are highly effective in the real world.
Along with this, there have been observational studies that show the vaccines have reduced the amount of transmission and need for hospitalization [9, 23]. Through a recent study by the Center of Disease Control and Prevention (CDC), it was concluded that the “SARS-CoV-2 vaccines significantly reduce the risk for COVID-19–associated hospitalization in older adults and, in turn, might lead to commensurate reductions in post-COVID conditions and deaths.” [9]
The vaccines have created an opportunity for the world to return to a somewhat normal reality through the concept of herd immunity. Herd immunity is the idea that a “large portion of a community becomes immune to a disease … As a result, the whole community becomes protected—not just those who are immune” [30]. In other words, as more people get vaccinated, the transmissibility of SARS-COV-2 will be significantly reduced. Proof of this comes from the CDC as they discovered that in 1000 working days, infections among unvaccinated individuals (1.38 infections) were significantly higher than both fully vaccinated (0.04 infections) and partially vaccinated individuals (0.19 infections) [10]. To put it simply, the COVID-19 vaccine works. The vaccine has protected individuals throughout the past 6 months, and now that it is readily available we are seeing a massive decline in cases [27].
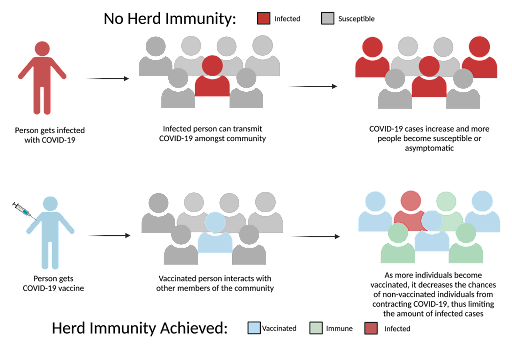
Figure 2. This diagram demonstrates how herd immunity functions in our society. As shown, the more people that are vaccinated, they are less likely to become infected.
Versatility:
Researchers have started studying possible applications of mRNA vaccines to diseases such as AIDS and other incurable diseases. It has been difficult to make regular vaccines due to the fact that there are so many mutations and strains, however the mRNA vaccine has been able to sidestep that by teaching the body to make antibodies and proteins. Before the modern advancements of mRNA vaccines that the COVID-19 vaccine brought forward, there was no efficient and effective way to deliver mRNA into the cell [31-32]. According to Mu et al. until these recent developments, there were major bottlenecks that hindered such research because mRNA is very unstable and can easily denature [31-32]. With new research, Moderna has begun trials on various mRNA vaccines, including one for HIV and AIDS [29].
Along with HIV, there has been research into using mRNA vaccines to treat cancer. Two types of vaccines have been proposed for cancer: preventative vaccines and treatment vaccines. Preventative vaccines attempt to protect the body from viruses that can potentially lead to cancer. HPV and Hepatitis B are two infections where vaccines have been made in an effort to prevent these infections and stop the development of cancer [43]. In this method, the body “mount[s] an attack against cancer cells … Instead of preventing disease, they are meant to get the immune system to attack a disease that already exists” [43]. Treatment based vaccines, meanwhile, are more personalized to an individual’s genome [49]. To implement this, there must be an understanding of the individual’s specific cancer genome [49]. Scientists identify the mutated genes that are responsible for the tumor growth in the individual. They then encode and inject the mutant mRNA into the body, providing the individual’s immune system with instructions to create the mutated protein. This mRNA enables the body to identify and attack the cells with markers for the mutated gene, which are not present in non-cancerous cells. Moderna implemented a similar approach and found that the method reduced tumor size in 30% of human participants when combined with checkpoint inhibitors, a drug which activates proteins to regulate the immune system when attacking cancer cells [49, 54]. Through the use of an mRNA vaccine, this allows the body to fight the tumors on its own rather than using harsh chemical mixtures, like chemotherapy, to stop the growth of the cancer.
In regards to the multitude of other infectious diseases, much of the research around mRNA vaccines has already started and will continue. With the full approval of the Pfizer vaccine and current EUA of Moderna, the opportunity for future mRNA vaccines seems promising. As noted in previous research for mRNA vaccines targeting Zika and other diseases, there was a lack of knowledge regarding mRNA vaccines that impacted the ability to create a successful vaccine [19]. Due to the recent advancements, the opportunity to revisit these vaccines is possible.
Limitations:
Several major hurdles continue to limit the broad application of mRNA vaccines which include cost, safety concerns, and instability of mRNA affecting storage.
Cost:
Due to the severity of COVID-19, funding was readily available in an effort to mitigate the spread of this deadly virus. The federal government was one of the major financial suppliers as they “pledged to give nearly $500 million to Moderna alone for its COVID-19 vaccine”, and this was able to support one of the first COVID-19 vaccines brought forward [24]. Dr. Nathaniel Wang, chief executive of Replicate Bioscience developing RNA-based treatments for cancer, said “it’s pretty hard to talk people into taking bets on this type of technology for vaccines in infectious diseases” because it is seen as “new” technology [19]. This has been gravely apparent regarding RNA vaccines for diseases like Zika [19]. These financial constraints delayed progress and it made mRNA vaccines a nonviable strategy of treatment for Zika, COVID, and other diseases previously discussed.
Safety:
The safety concerns regarding the COVID-19 vaccine have been particularly contentious in the U.S. This fear is fueled by misinformation such as rumors of infertility caused by the vaccine and other false claims that have been reported in opinion pieces online. Many of the conspiracy theories and stories that damaged the image of the vaccine originated from social media[21]. A study polled that a majority of Americans believe there was “rushed approval for the COVID-19 vaccine without the assurances of safety and efficacy” causing people to believe that the vaccine bypassed all the regulatory steps [22]. The FDA defines that “for an EUA to be issued for a vaccine… FDA must determine that the known and potential benefits outweigh the known and potential risks of the vaccine” [39]. Through years of advanced research, the trials and production of the vaccine were able to run in parallel without compromising the safety of the vaccine [50]. While there are some valid concerns specific to the COVID-19 mRNA vaccines, including myocarditis, blood clots, and potential allergic reactions, the COVID-19 mRNA vaccines have been deemed as safe and effective by the CDC [26].
Side effects:
It is possible that individuals will experience certain side effects ranging from pain, swelling in the arm, nausea and fever, along with some more serious side effects, for example myocarditis and blood clots, reported by the CDC. It is important to note that if these less serious side effects even occur they are generally present for less than a week. A small price to pay for a vaccine that has been effective in preventing the spread of COVID-19 [23]. This was shown through mouse and hamster trials, as they noted that they had full immune system responses that protected against COVID-19, similar to that of humans [57]. In another study done with rats, they focused on the vaccine’s potential impact on pregnant rats to simulate that of a pregnant woman and found that there are potential side effects on that impact fetal development, female fertility, and early offspring development, but none were observed [58].
Through a variety of trials, scientists have determined that the body has been able to perform a timely immune response to the vaccine. A measurement of this has been the body’s reaction in the form of specific side effects [52]. Only a small number of cases include more serious reactions, such as anaphylaxis (2.5 per 1 million Moderna vaccines). Most cases will only have small reactions and no long-term side effects have been recorded [34, 35]. Though the majority of people only have minor reactions, these side effects show that the vaccine has gotten into the cell and the body has identified the viral mRNA [52].
Through the immense amount of data showing the vaccine’s efficacy, Pfizer has received FDA approval while Moderna has begun the FDA approval process [36, 37]. This milestone highlights the safety and efficacy of both mRNA vaccines.
Storage:
Due to the fact that both Moderna and Pfizer need lower temperatures for stability, they require the vaccines be kept below freezing around -20 to -80 degrees C for long term storage [25]. RNA needs to be stored at lower temperature as it will degrade due to alkaline hydrolysis, (breakdowns on its own in basic conditions) and RNAse activity (a nuclease that cleaves RNA). There have been cases of COVID-19 vaccines being discarded due to improper storage [55]. This limits packaging, shipment, and regions of the world allowed to have access to these vaccines because their storage will require specialized equipment and refrigeration.
Conclusion:
The COVID-19 vaccines have paved a way for more mRNA vaccines to be brought to the medical field. If there is a steady increase in funding, researchers can begin to establish these kinds of vaccines for a variety of different diseases. By working through setbacks and finding a way to deliver vaccinations to the masses as well as bringing money to research, many of the limitations of mRNA vaccines can be mitigated in the future. The COVID-19 vaccine has proven to be quite efficacious and the recent FDA approvals are evident of this. These vaccines have been able to set a precedent of how mRNA vaccines can be used throughout health care as a protective measure. mRNA vaccines are still considered a “new” technology and will continue to be researched and applied to a wide variety of fields in the future.
References:
- Cobb, Matthew. “Who discovered messenger RNA?.” Current Biology 25, no. 13 (2015): R526-R532.
- Brenner, Sydney, et al. “An unstable intermediate carrying information from genes to ribosomes for protein synthesis.” Nature 190, no. 4776 (1961): 576-581.
- Gros, François, et al. “Unstable ribonucleic acid revealed by pulse labeling of Escherichia coli.” Nature 190, no. 4776 (1961): 581-585.
- Jacob, François, and Jacques Monod. “Genetic regulatory mechanisms in the synthesis of proteins.” Journal of molecular biology 3, no. 3 (1961): 318-356.
- Yčas, Martynas, and W. S. Vincent. “A ribonucleic acid fraction from yeast related in composition to desoxyribonucleic acid.” Proceedings of the National Academy of Sciences of the United States of America 46, no. 6 (1960): 804.
- Novelli, G., Biancolella, M., Mehrian-Shai, R. et al. COVID-19 one year into the pandemic: from genetics and genomics to therapy, vaccination, and policy. Hum Genomics 15, 27 (2021). https://doi.org/10.1186/s40246-021-00326-3
- “Understanding MRNA COVID-19 Vaccines.” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/mrna.html.
- Khubchandani, Jagdish, et al. “COVID-19 Vaccination Hesitancy in the United States: A Rapid National Assessment.” Journal of Community Health, Springer US, 3 Jan. 2021, link.springer.com/article/10.1007/s10900-020-00958-x.
- “Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Among Hospitalized Adults Aged ≥65 Years – United States, January–March 2021.” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, 6 May 2021, www.cdc.gov/mmwr/volumes/70/wr/mm7018e1.htm?s_cid=mm7018e1_w.
- “Interim Estimates of Vaccine Effectiveness of BNT162b2 and MRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Health Care Personnel, First Responders, and Other Essential and Frontline Workers – Eight U.S. Locations, December 2020–March 2021.” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, 1 Apr. 2021, www.cdc.gov/mmwr/volumes/70/wr/mm7013e3.htm?s_cid=mm7013e3_w.
- Bringmann, A., et al. “RNA Vaccines in Cancer Treatment”, BioMed Research International, vol. 2010, Article ID 623687, 12 pages, 2010. https://doi.org/10.1155/2010/623687
- Miao, L., Zhang, Y. & Huang, L. mRNA vaccine for cancer immunotherapy. Mol Cancer 20, 41 (2021). https://doi.org/10.1186/s12943-021-01335-5
- WHO. How do vaccines work? (2020, December 8). World Health Organization. https://www.who.int/news-room/feature-stories/detail/how-do-vaccines-work
- What are nucleic acid vaccines and how could they be turned against COVID-19? (n.d.). GAVI.https://www.gavi.org/vaccineswork/what-are-nucleic-acid-vaccines-and-how-could-they-be-used-against-covid-19
- Pardi, N., Hogan, M., Porter, F. et al. mRNA vaccines — a new era in vaccinology. Nat Rev Drug Discov 17, 261–279 (2018). https://doi.org/10.1038/nrd.2017.243
- Understanding mRNA COVID-19 Vaccines. (2021, March 4). CDC. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/mrna.html
- Huang Q, Zeng J, Yan J. COVID-19 mRNA vaccines. J Genet Genomics. 2021;48(2):107-114. doi:10.1016/j.jgg.2021.02.006 https://www.sciencedirect.com/science/article/abs/pii/S167385272100045X
- U.S. Department of Health and Human Services Food and Drug Administration Center for Biologics. Evaluation and Research. (n.d.). Development and Licensure of Vaccines to Prevent COVID-19 Guidance for Industry. FDA. https://www.fda.gov/media/139638/download
- Dolgin, E. (2021, January 12). How COVID unlocked the power of RNA vaccines. Nature. https://www.nature.com/articles/d41586-021-00019-w#ref-CR3
- Zhang, C., Maruggi, G., Shan, H., & Li, J. (0001, January 01). Advances in mRNA Vaccines for Infectious Diseases. Retrieved from https://www.frontiersin.org/articles/10.3389/fimmu.2019.00594/full
- Sriskandarajah, I. (2021, June 5). WHERE DID THE MICROCHIP VACCINE CONSPIRACY THEORY COME FROM ANYWAY? The Verge. https://www.theverge.com/22516823/covid-vaccine-microchip-conspiracy-theory-explained-reddit
- Khubchandani, J., Sharma, S., Price, J.H. et al. COVID-19 Vaccination Hesitancy in the United States: A Rapid National Assessment. J Community Health 46, 270–277 (2021). https://doi.org/10.1007/s10900-020-00958-x
- CDC. (2021, September 15). Science Brief: COVID-19 Vaccines and Vaccination. CDC. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/fully-vaccinated-people.html
- Rosenbaum, L. (2020, May 09). Fueled By $500 Million In Federal Cash, Moderna Races To Make A Billion Doses Of An Unproven Cure. Retrieved from https://www.forbes.com/sites/leahrosenbaum/2020/05/08/fueled-by-500-million-in-federal-cash-moderna-races-to-make-1-billion-doses-of-an-unproven-cure/?sh=54dbd69279dc
- Crommelin, D. J. A., et al. (2020, December 11). Addressing the Cold Reality of mRNA Vaccine Stability. Journal of Pharmaceutical Sciences. Retrieved September 17, 2021, from https://jpharmsci.org/article/S0022-3549(20)30785-1/fulltext#relatedArticles.
- Is the COVID-19 Vaccine Safe? (n.d.). Retrieved from https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/is-the-covid19-vaccine-safe
- Have we flattened the curve in California? – Johns Hopkins. (n.d.). Retrieved from https://coronavirus.jhu.edu/data/new-cases-50-states/california
- Chuck, E. (2021, July 06). They didn’t want to get Covid-19 shots. This is what convinced them. Retrieved from https://www.nbcnews.com/news/us-news/they-didn-t-want-get-covid-19-shots-what-convinced-n1272740
- Chodosh, S. (2021, August 18). The first mRNA-based HIV vaccine is about to start human trials. PopSci. https://www.popsci.com/health/moderna-mrna-hiv-vaccine/
- Herd immunity and COVID-19 (coronavirus): What you need to know. (2021, August 28). Retrieved from https://www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/herd-immunity-and-coronavirus/art-20486808
- HIV mRNA Vaccine Platforms. (n.d.). Retrieved from https://encyclopedia.pub/12314
- Mu Z, Haynes BF, Cain DW. HIV mRNA Vaccines-Progress and Future Paths. Vaccines (Basel). 2021 Feb 7;9(2):134. doi: 10.3390/vaccines9020134. PMID: 33562203; PMCID: PMC7915550.
- Messenger RNA (mRNA). (n.d.). Retrieved from https://www.genome.gov/genetics-glossary/messenger-rna
- CDC. Possible Side Effects After Getting a COVID-19 Vaccine. (n.d.). Retrieved from https://www.cdc.gov/coronavirus/2019-ncov/vaccines/expect/after.html
- CDC. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Moderna COVID-19 Vaccine – United States, December 21, 2020–January 10, 2021. (2021, January 28). Retrieved from https://www.cdc.gov/mmwr/volumes/70/wr/mm7004e1.htm
- Commissioner, O. O. (n.d.). FDA Approves First COVID-19 Vaccine. Retrieved from https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine
- Billingsley, A. (2021, September 15). FDA Approval Updates: Pfizer, Moderna, and J&J COVID-19 Vaccine – GoodRx. Retrieved from https://www.goodrx.com/blog/fda-covid-19-vaccine-approval-updates/
- Heinz, F.X., Stiasny, K. Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. npj Vaccines 6, 104 (2021). https://doi.org/10.1038/s41541-021-00369-6
- FDA.Center for Biologics Evaluation and Research. (n.d.). Emergency Use Authorization for Vaccines Explained. Retrieved from https://www.fda.gov/vaccines-blood-biologics/vaccines/emergency-use-authorization-vaccines-explained
- Ritchie, H., et al. (2020, March 05). Coronavirus (COVID-19) Vaccinations – Statistics and Research. Retrieved from https://ourworldindata.org/covid-vaccinations?country=USA
- Santhanam, L. As more Americans get vaccinated, 41% of Republicans still refuse COVID-19 shots. (2021, May 17). Retrieved from https://www.pbs.org/newshour/amp/health/as-more-americans-get-vaccinated-41-of-republicans-still-refuse-covid-19-shots
- CDC. HIV Treatment. (2021, May 20). Retrieved from https://www.cdc.gov/hiv/basics/livingwithhiv/treatment.html
- Cancer Vaccines and Their Side Effects. (n.d.). Retrieved from https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/immunotherapy/cancer-vaccines.html
- Fiedler K, et al. mRNA Cancer Vaccines. Recent Results Cancer Res. 2016;209:61-85. doi: 10.1007/978-3-319-42934-2_5. PMID: 28101688. https://pubmed.ncbi.nlm.nih.gov/28101688/
- Ulmer J.B., Geall A.J. Recent innovations in mRNA vaccines. Curr. Opin. Immunol. 2016;41:18–22.
- Pardi N., et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature. 2017;543:248–251.
- Maruggi G., et al. mRNA as a transformative technology for vaccine development to control infectious diseases. Mol. Ther. 2019;27:757–772.
- MRNA Vaccines Development. (n.d.). Retrieved from https://mrna.creative-biolabs.com/mrna-vaccines-development.htm?gclid=CjwKCAjw4qCKBhAVEiwAkTYsPF7-OmStgBDEDy6_ksYSAc6XFrOTMOqn9rnCOLblUh4K4LX3vX18LhoCQ3AQAvD_BwE
- How mRNA Vaccines Help Fight Cancer Tumors, Too – Penn Medicine. (n.d.). Retrieved from https://www.pennmedicine.org/news/news-blog/2021/june/how-mrna-vaccines-help-fight-cancer-tumors-too
- Ball, P. (2020, December 18). The lightning-fast quest for COVID vaccines – and what it means for other diseases. Retrieved from https://www.nature.com/articles/d41586-020-03626-1
- MRNA and the future of vaccine manufacturing. (n.d.). Retrieved from https://www.path.org/articles/mrna-and-future-vaccine-manufacturing/
- Side Effects of COVID-19 Vaccines. (n.d.). Retrieved from https://www.who.int/news-room/feature-stories/detail/side-effects-of-covid-19-vaccines
- What is the difference between efficacy and effectiveness? Gavi, the Vaccine Alliance. (2020, November 18). Retrieved October 23, 2021, from https://www.gavi.org/vaccineswork/what-difference-between-efficacy-and-effectiveness.
- Checkpoint inhibitors. Checkpoint inhibitors | Types of immunotherapy | Cancer Research UK. (2021, May 19). Retrieved October 23, 2021, from https://www.cancerresearchuk.org/about-cancer/cancer-in-general/treatment/immunotherapy/types/checkpoint-inhibitors.
- Levin, D. (2021, August 1). The U.S. is wasting vaccine doses, even as cases rise and other countries suffer shortages. The New York Times. Retrieved October 23, 2021, from https://www.nytimes.com/2021/08/01/us/covid-us-vaccine-wasted.html.
- Myhre, J., & Sifris, D. (2021, August 2). Why is it so hard to make an HIV vaccine? Verywell Health. Retrieved January 19, 2022, from https://www.verywellhealth.com/hiv-vaccine-development-4057071
- Cagigi, A., & Loré, K. (2021). Immune Responses Induced by mRNA Vaccination in Mice, Monkeys and Humans. Vaccines, 9(1), 61. https://doi.org/10.3390/vaccines9010061
- Anand, P., Stahel, V.P. The safety of Covid-19 mRNA vaccines: a review. Patient Saf Surg 15, 20 (2021). https://doi.org/10.1186/s13037-021-00291-9
