By Chengyu Sun, Evolution, Ecology, and Biodiversity, ’25
Author’s Note:
This review article was written for the assignment of Dr. Brenda Rinard’s UWP 102B. I chose the topic of discussing the behavior and uniqueness of Toxoplasma gondii because parasitic behavior really fascinates me and I would want to further study this area in the future. Initially I wanted to discuss multiple parasite’s behavior and the similarities but was told by Dr. Rinard to narrow down my topic to one species and I chose Toxoplasma gondii. Although the protozoan parasite does not cause much of a health problem in immunocompetent people, it does accomplish infecting more than one third of the entire world population. This review article aims not only to inform the scientific scholars of new approaches in studying this parasite or studying different species, but also could function as a general education article to let more people know the disease and understand its functions.
Abstract
Infection of Toxoplasma gondii within intermediate hosts is not virulently fatal but cognitively concerning. They usually occur within muscle cells and the central nervous system, which could be the explanation for multiple behavior alterations observed in hosts. Through experiments done on infected lab rats, scientists found that innate behaviors of neophobia and escaping from predators were transformed by a single infection. Infections in humans are commonly described as flu-like symptoms and are not lethal unless the patient is immunodeficient or the parasite was transmitted from mother to child during pregnancy (McAuley 2014; Martorelli Di Genova et al. 2019). Infected human patients have shown more outgoing or violent personality changes depending on one’s sex (Flegr and Hrdý 1994). Flegr and Hrdý showed that the parasite causes infection, and infection results in abnormal personality changes associated with mental illness such as schizophrenia and bipolar disease. The same antipsychotic medication and mood stabilizer used to treat schizophrenia and bipolar disease had slowed the infection of T.gondii.
Introduction
Toxoplasma gondii is a protozoan parasite that evolved to infect cat hosts but has the capability of being transmitted to all mammalian animals and making them their intermediate hosts (Dubey et al. 2012). In fact, at least one-third of the world’s human population has been infected by this protozoan. In some areas like Brazil, at least 50% of the population have antibodies toward this parasite due to the highly contaminated environment containing significant levels of oocysts, a stage of the parasite that is often found in host feces and produces infectious spores if ingested. (Dubey et al. 2012; Attias et al. 2020). As a model of the intermediate host, infected rodents are surprisingly prone to the odor of cat urine and identify the smell as “attractive”, while uninfected rodents would always escape from an area with cat smells. This difference in behavior makes the infected more likely to be eaten by cats thus helping the parasites to enter into cats’ intestines (Webster 2007).
Humans are considered to be dead-end hosts due to the low probability of someone being eaten by any feline species, however, the parasite infection would be concentrated mostly in the central nervous system where they could alter the neurotransmitter secretion concentration, mainly dopamine, further leading to obvious personality change. The personality change often accompanies risky behavior or outgoing characteristics and an increase in the probability of congenital transmission to infants (Attias et al. 2020).
Even though research on T.gondii has just begun in the past hundred years, this parasite has coevolved with nature since domestic cats first appeared in history—their DNA even appearing in the mummies of ancient Egypt (Khairat et al. 2013). These thousands of years of coevolution with all its hosts provided this parasite with the ability to manipulate each of them. There is certainly far more knowledge of this parasite that is unknown to us. In this review, we will see how current science unravels the mystery of how T.gondii manipulates host behavior.
Intestinal
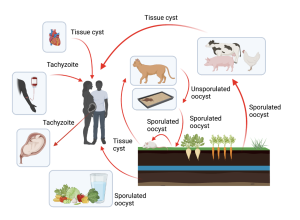
Figure 1: Transmission of T.gondii could involve multiple intermediate hosts primarily through ingesting contaminated food sources.
Delta-6-desaturase: why cats?
We first must address the elephant in the room, that is why cats? Why did T. gondii choose the feline as its definitive host when it has the ability to infect almost all other mammals? It turns out that linoleic acid secretion is the key.
As it turns out, cats are the only mammal that secretes linoleic acid in their body since they don’t carry the enzyme delta-6-desaturase in its small intestine. This enzyme decomposes intestinal linoleic acid in all other species, but since cats don’t have the enzyme, their linoleic acid levels remain very high. (Martorelli et al. 2019). Martorelli et al. (2019) mimicked the intestinal environment of a cat to determine whether this difference makes cats ideal for sexual reproduction. The scientists shredded cat intestine epithelial cells onto glass coverslips to best replicate the favorable environments for parasite reproduction rather than in vivo experiments. The experiment utilizes two cat intestine models; the control group has no amino acids attached while the test group intestine model has linoleic acid and oleic acid supplements which have almost identical molecular structure but one double bond difference. To detect T. gondii, they used merozoite markers, a detection method that finds amoeboids capable of reproduction. They found that the man-made intestine model with a large amount of linoleic acid successfully cultured T. gondii while the control groups did not show signs of sexual reproduction.
With this discovery, they proposed another hypothesis: if the delta-6-desaturase pathway in infected mice is shut down using a chemical named SC-26196, would the mice obtain a high level of linoleic acid and thus induce sexual reproduction of T. gondii in their intestine? A followup experiment found that merely SC-26196 alone couldn’t help the parasite reach the reproductive stage in mice. Instead, both the chemical and additional linoleic acid were needed, and even then the generated oocysts could not infect other live mice well. This means that T. gondii also needs optimal physiological conditions such as a stable body temperature, and ideal microbiome inside the intestines to enable access to the linoleic acid. The study also indicated that the absence of delta-6-desaturase doesn’t always make the ideal environment for sexual reproduction: extremely high concentrations of linoleic acid is also toxic for tachyzoites (T. gondii’s rapid replication phase during early infection).
Lastly, Martorelli et al. found that linoleic acid is likely to function as a signaling molecule instead of nutrition for the parasite because of its similarity to oleic acid with only one double bond difference, making it less likely a reaction material. The species barrier for the sexual development of T. gondii could be broken but there is no evidence that the parasite would alter the intermediate hosts’ intestine culture, at least so far.
Infection in rodents—behavior manipulation towards fearless action
We’ve now established that T.gondii can’t accomplish sexual reproduction in mice, so they have to make their way out of the rodent and into the body of a cat. According to the manipulation hypothesis, parasites increase the transmission rate of themselves by controlling the intermediate host’s behavior to have a higher chance of being eaten by the definitive host. T. gondii achieved this goal with three distinct actions, (1) increase in infected species movement and exploring activity (less periods of stillness resulting in decreased surveillance behaviors), (2) decrease in neophobia (fear of novelty), and (3) impairment of cognitive perception in face of predation risk. However, scientists still don’t know the pathological origin of these abnormal behaviors (Berdoy et al. 2000; Webster 2007). Berdoy et al. (2000)’s experiment includes lab rats, hundreds of generations without getting in contact with cats. The scientists placed them in maze-like pens with 16 cells, presenting multiple odors including control groups and cat’s urine odor. The T. gondii-infected rats showed great attraction to the cat odor and spent most of their time exploring the cells and staying active during the night while uninfected rats performed great aversion towards its natural predator’s scent. Their responses towards other smells were evidently similar with uninfected rats, indicating that the parasites do not impair their olfactory faculties but instead control their evolutionary behavior against predators. At the same time, similar “fearless” actions in rats are found in the ones given anti-anxiety drugs, proposing a similar pathway between manipulation and anxiety control mechanisms (Berdoy et al. 2000).
Another study on T.gondii’s influence on rodent behavior noted that even though the high infection rate in their subjects’ brains contributes to this phenomenon (reduction in neophobia and increased fearlessness towards predators), whether the specific alternation happens neurologically or immunologically is still unknown (Webster 2007). By reviewing research in human schizophrenia drug tests and Webster’s own previous research, she proposed another possible pathway for control that is similar to the anxiety-repressing technique. By blocking the host’s anxiogenic N-methyl-D-aspartic acid receptors in the amygdala and providing serotonin antagonists that could make the rats disregard danger, Webster found a possible connection with schizophrenia’s roots, pointing out a new possible direction in treating the disease (2007). This specific operation in neurological and psychological behavior could be significantly observed in human hosts.
Infection in humans—health risk and personality alternation towards more interactions
Since human beings are also part of the infection cycle of T.gondii, Flegr and Hrdý (1994) believe the alternation of behavior seen in rodents and other species of hosts could also be seen in humans, especially personality changes towards exposure to danger. By obtaining data from 195 male and 143 female subjects experiencing chronic infection of T. gondii over a period of more than a year and using Cattell’s sixteen factor questionnaire (16PF) to generate the results, they discovered more significant personality differences in infected and healthy male subjects. In particular the results showed infected men are more schizothymic (detached and critical), have lower superego strength (disregards rules) while self-sentiment strength is low and experience more pretension (suspecting and jealousy). However the data collection might have been biased since the tested subjects were all collected from Charles’ University’s Faculty of Science (Flegr and Hrdý 1994). Among the infected male subjects, one could deduce that several factors are interrelated like schizothymia and low self-sentiment, and are thus likely caused by pretension and low superego rather than the parasite. Thus, the link between T. gondii and personality alteration still requires more research.
Flegr conducted another study in 2007 that examines broader human behaviors. This time, Flegr had his participants go through 16PF and Cloninger’s Temperament and Character Inventory (TCI) personality test. This research is more reliable than the previous study since it includes more diverse samples from not only the university departments but also military personnel and blood donors, making the result more widely applicable. For the results, infected men tended to be more violent and more likely to ignore rules. Infected women showed a higher superego, becoming more outgoing and warmhearted and having higher apprehension compared with their healthy counterparts. Latently infected individuals had significantly longer response times to approaching danger and more easily lost their attention, making them 2.65 times more likely to have a traffic accident either as the pedestrian or the driver (Flegr 2007). Just like in rodents, we see that T. gondii impairs motor performance in humans as well.
Symptoms in infected patients are not the only problems worth concerning; T. gondii is also capable of infecting the fetus of a pregnant mother. In an earlier 2005 study, Flegr et al. examined the health impacts of T. gondii on pregnant women, using 16-week pregnancy individuals and discarding high-antibody subjects due to insufficient infection period. They found that early infection decreases the body weight of pregnant women and slows or stunts fetus development. Ultrasonography tests at 16-weeks pregnancy may underestimate the severity T.gondii has on the weight of pregnant women.
Psychological illnesses, their relationship to T. gondii infection, and possibly effective treatment:
T.gondii infection and personality and behavior change can be further linked to symptoms of neurological disorders such as bipolar disorder and the aforementioned schizophrenia, as the infection could do damage to serotonin secretion and lead to such psychological illnesses. But how can we distinguish parasite-induced mental illnesses from regular psychological abnormalities? In order to find out the solution to this, scientists looked back at the rodents to find some possible treatments. Webster et al. (2006), for example, recycled their neophobic mice experiment setup for another study on whether three medications, HAL (an antipsychotic), VAL (a mood-stabilizer), and an anti-parasite medication called PD, would affect treated subjects. They tested all three treatments on both infected rats and non-infected rats separately (with non-treated infected and uninfected rats as control groups) to test their activity level, feline attraction, and alertness.
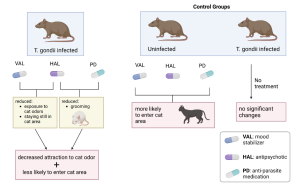
Figure 2: Drugs treating psychotic diseases of humans used on rodents show alleviation of infection, but adverse effects appear when these drugs are used to treat uninfected rodents, suggesting careful drug use and correct diagnosis before treatment.
The results showed that all three treatments significantly decreased the rats’ attraction to cat odor and decreased their likelihood of entering the cat area by almost 200% compared to the control groups. But the duration of their stay in the cat area decreased insignificantly. HAL and VAL treatments significantly reduced the time spent on exposure to cat smells and staying still in the cat area by 75% and 50% respectively, while HAL and PD significantly reduced the time spent on grooming by 60%. However, the medication used on uninfected rats made them more likely to enter the cat area and increased their duration of stays there, suggesting caution is necessary when assigning these medications to humans in order to avoid negative effects making the infection worse.
Finally, the main focus of medication should be aimed at the treatment of T. gondii infection in humans. Jones et al. (2003) tried to explore how antipsychotics and mood stabilizers would contribute to the inhibition of T.gondii infection as multiple antipsychotic medications have been found to have a certain level of antiprotozoal activity in patients.
They tested the effect of 8 antipsychotics and 4 mood stabilizers on both parasite and host cells. Each medication was ranked using a therapeutic index (TI)—the higher the value, the safer and better the drug. For example, the 12.1 TI value for trimethoprim, a commonly used drug for the treatment of T.gondii in humans, is similar to the 13.9 TI value of valproic acid. When these two drugs were tested together on their effect against T. gondii, the TI reached 39.5, while the concentration of trimethoprim and valproic acid alone in this treatment were much lower than the prescription level one patient would receive from their doctor , suggesting significant synergistic effects. The findings also show that the normal treatment of valproic acid for schizophrenic and bipolar patients is way above the tested threshold that the medication would be effective in inhibiting T.gondii infection (Jones-Brando 2003). However, as Webster (2006) observed uninfected mice developing infected-like behavior under treatment, antipsychotics and mood stabilizers should nevertheless be prescribed with great caution.
Conclusion
The protozoan parasite Toxoplasma gondii was found around 100 years ago. Although we have reliable evidence on how the parasite infects a host and the possible consequences for different hosts including rodents and humans, there are still lots of unknown aspects of this parasite such as personality manipulation (Webster, 2007). As human development over the past hundreds years was profoundly significant, determining when the parasite gained the ability to infect humans is also an important component of unlocking this protozoan’s learning mechanism to help tackle worldwide infections.
Works Cited
- Attias M, Teixeira DE, Benchimol M, Vommaro RC, Crepaldi PH, De Souza W. 2020. The life-cycle of Toxoplasma gondii reviewed using animations. Parasites Vectors. 13(1):588. doi:10.1186/s13071-020-04445-z.
- Berdoy M, Webster JP, Macdonald DW. 2000. Fatal attraction in rats infected with Toxoplasma gondii. Proc R Soc Lond B. 267(1452):1591–1594. doi:10.1098/rspb.2000.1182.
- Dubey JP, Lago EG, Gennari SM, Su C, Jones JL. 2012. Toxoplasmosis in humans and animals in Brazil: high prevalence, high burden of disease, and epidemiology. Parasitology. 139(11):1375–1424. doi:10.1017/S0031182012000765.
- Dubey JP, Lindsay DS, Speer CA. 1998. Structures of Toxoplasma gondii Tachyzoites, Bradyzoites, and Sporozoites and Biology and Development of Tissue Cysts. Clin Microbiol Rev. 11(2):267–299. doi:10.1128/CMR.11.2.267.
- Flegr J. 2007. Effects of Toxoplasma on Human Behavior. Schizophrenia Bulletin. 33(3):757–760. doi:10.1093/schbul/sbl074.
- Flegr J, Hrdá Š, Kodym P. 2005. Influence of latent “asymptomatic” toxoplasmosis on body weight of pregnant women. FOLIA PARASIT. 52(3):199–204. doi:10.14411/fp.2005.026.
- Flegr J, Hrdý I. 1994. Influence of chronic toxoplasmosis on some human personality factors. Folia Parasitol (Praha). 41(2):122–126.
- Jones-Brando L. 2003. Drugs used in the treatment of schizophrenia and bipolar disorder inhibit the replication of Toxoplasma gondii. Schizophrenia Research. 62(3):237–244. doi:10.1016/S0920-9964(02)00357-2.
- Khairat R, Ball M, Chang C-CH, Bianucci R, Nerlich AG, Trautmann M, Ismail S, Shanab GML, Karim AM, Gad YZ, et al. 2013. First insights into the metagenome of Egyptian mummies using next-generation sequencing. J Appl Genetics. 54(3):309–325. doi:10.1007/s13353-013-0145-1.
- Martorelli Di Genova B, Wilson SK, Dubey JP, Knoll LJ. 2019. Intestinal delta-6-desaturase activity determines host range for Toxoplasma sexual reproduction. Striepen B, editor. PLoS Biol. 17(8):e3000364. doi:10.1371/journal.pbio.3000364.
- McAuley JB. 2014. Congenital Toxoplasmosis. Journal of the Pediatric Infectious Diseases Society. 3(suppl_1):S30–S35. doi:10.1093/jpids/piu077.
- Webster JP. 2007. The Effect of Toxoplasma gondii on Animal Behavior: Playing Cat and Mouse. Schizophrenia Bulletin. 33(3):752–756. doi:10.1093/schbul/sbl073.
- Webster JP, Lamberton PHL, Donnelly CA, Torrey EF. 2006. Parasites as causative agents of human affective disorders? The impact of anti-psychotic, mood-stabilizer and anti-parasite medication on Toxoplasma gondii ’s ability to alter host behaviour. Proc R Soc B. 273(1589):1023–1030. doi:10.1098/rspb.2005.3413.

