By Sabrina Lazar, Cell Biology ‘20
Author’s note: After attending an interesting meeting on cytoskeleton dynamics in the weekly Joint Seminars in Molecular Biology series, I wanted to learn more about the subject and found Anna Franz and her colleagues’ recent paper about fat cells in Drosophila, a model organism I work with and is dear to me. This essay serves as a way for me to share fascinating research with those that are interested in Drosophila, cells, or biology in general.
In general, those with some knowledge of biology will explain that fat tissue exists to store energy. However, science has progressed in recent years with the discovery of previously unknown functions of common tissue types. In this vein, a new paper surfaced in the February 2018 issue of Developmental Cell titled “Fat Body Cells are Motile and Actively Migrate to Wounds to Drive Repair and Prevent Infection.” In their experiment, Franz et al sought to learn all about how adipocytes, also referred to as fat body cells, function at microscopic laser wound sites in Drosophila. Anna Franz, the first author of the paper, outlines the current state of fat research, saying there is a “growing realization that adipocytes… have numerous other roles in various tissues in health and disease” [2].
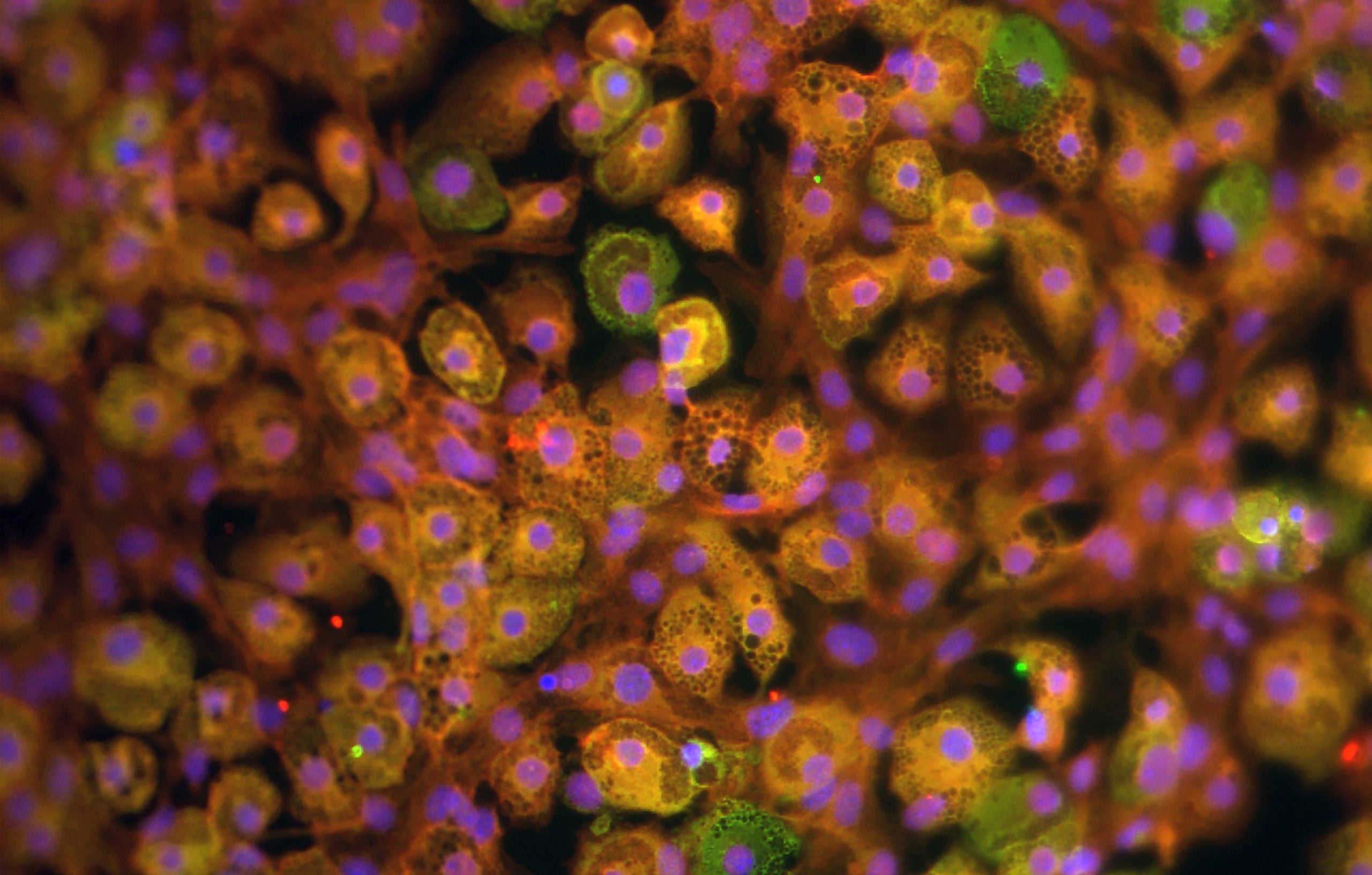
The team found that the adipocytes are capable of motion and move towards gaps in the external epithelial tissue, which can be thought of as open wounds. To create these gaps, a fine laser was used to create small epithelial wounds in the flies’ ventral thorax, an area with very low adipocyte density. This tested if cells could first travel to this distant spot and then would track their behavior at the target location. Franz reports the cells were “highly dynamic” in their journey, and that once there, the cells “remained tightly associated with the wound” and remained there until the broken epithelium was restored [2]. To ensure this was not a random motion driven by passive fluid, the team tracked individual cells under fluorescence microscopy and saw the cells move independently in the direction of the wound. Tagging specific proteins involved in cellular movement with colorful fluorescent molecules allowed the researchers to observe and track fat body cells traveling and coordinating to the open space as time progressed.
The team also used live fluorescent imaging of the actin proteins in the cytoskeleton to understand how exactly the adipocyte movement mechanism worked. What they found was a undulation of actin strands from the cell cortex; the long actin fibers comprising the adipocyte’s cytoskeleton were oscillating in a wave-like motion, driving the entire cell in the opposing direction of the waves.
By elucidating the adipocytes’ activities once they swam toward the cut, the team brought forth the next advancement. The researchers found that hemocytes in Drosophila— similar to macrophages, cells that defend the body by destroying bacteria and harmful particles— and adipocytes coordinate at the wound. It was already known that hemocytes help clear debris from the wound entrance [1], but when the hemocytes were inhibited, the team saw that the debris continued to be swept away, but by adipocytes. The observation exhibited a possible association between the immune system and fat body cells.
Since adipocytes are relatively large compared to other cells, the group hypothesized that they might play a role in sealing the wound gap to prevent infection. Examination under transmission electron microscopy (TEM) showed very close association between adipocytes and the broken epithelium, “leaving a gap of less than 20nm”[2]. Furthermore, fluorescently tagging actin regulatory proteins revealed that the adipocytes coordinating at the site extended membrane projections to reach around and out of the epithelial gap to seal off the gash.
Franz’s next steps are to branch out to other vertebrates to connect their findings with all kinds of eukaryotes, including humans. Other scientists have already started to branch out. Schmidt and Horsley found that mouse adipocytes function similarly to the ones in Drosophila in their 2013 paper [4]. In 2012, Osborn and Olefsky investigated “obesity-induced inflammation and insulin resistance”, and found that due to overnutrition, Drosophila adipocytes recruit macrophages to enter adipose (fat) tissue, which triggers inflammation and consequent insulin resistance [3]. Franz et al. concluded with the linkage between immune and fat body cells, and how they could be relevant to human diseases. Future research that looks more into the relationship between hemocytes and adipocytes can provide valuable insight into the cellular causes of diseases like Type II diabetes.
Works Cited
- B. Stramer, W. Wood, M.J. Galko, M.J. Redd, A. Jacinto, S.M. Parkhurst, P. Martin. “Live imaging of wound inflammation in Drosophila embryos reveals key roles for small GTPases during in vivo cell migration.” J. Cell Biol., 168 (2005), pp. 567-573
- Franz, Anna, Will Wood, and Paul Martin. “Fat body cells are motile and actively migrate to wounds to drive repair and prevent infection.” Developmental Cell 44.4 (2018): 460-470.
- Osborn, Olivia, and Jerrold M. Olefsky. “The cellular and signaling networks linking the immune system and metabolism in disease.” Nature medicine 18.3 (2012): 363.
- Schmidt, Barbara A., and Valerie Horsley. “Intradermal adipocytes mediate fibroblast recruitment during skin wound healing.” Development 140.7 (2013): 1517-1527.