By Madhulika Appajodu, Cell Biology ’24
Author’s Note: My name is Madhulika Appajodu and I am a 3rd Year Cell Biology major at UC Davis. I am a pre-medical student and hope to go on to medical school. I chose Cell Biology as a major because I found the focus on cell organization and function to be very interesting. I am a volunteer at Shifa Community Clinic and a member of MEDLIFE, SEND4C, and H4H. I am also a BioLaunch Mentor and a Learning Assistant for the Physics Department. I wrote this piece to answer the question: “How does prenatal nicotine exposure increase the risk of asthma in offspring?” I wrote this for undergraduate students in the field of epigenetics/prenatal exposures and experts/professors in the field but also for the general public who have some knowledge in science. I chose this topic in particular because epigenetics interests me greatly. I find that environmental factors likely play a large part in the life outcomes of people who may be genetically similar but grew up in different environments. I hope that readers will understand how important environmental factors are in the grand scheme of physical, emotional, and mental health for not just the reader but their future families (if they choose to have them) health as well.
ABSTRACT:
Previous studies have studied prenatal nicotine exposure and its effects which follow offspring over the course of their lives. One of these effects is asthma. Asthma is a chronic respiratory condition characterized by the narrowing of one’s airways in response to an allergen or irritant. It is a widespread condition, affecting over 25 million people currently in the US alone. The mechanisms of asthma and its causes are currently being investigated. However, researchers agree that prenatal nicotine exposure increases the risk of asthma in offspring exponentially.
There is currently no cure for asthma, only methods to lessen the intensity of asthmatic episodes, such as through the use of an inhaler. This literature review details the mechanisms through which prenatal nicotine exposure increases the risk of asthma in offspring, according to current research. The three potential causes of this increased risk are placental damage, epigenetic alteration, and nicotine exposure alone. The mechanisms will be evaluated through a synthesis of experimental and survey data in mice and human models in studies done in the past seven years. Comparisons will be drawn between articles that cite the same mechanism as the cause of the increased risk of asthma. Once the mechanism(s) are identified, research can be done to identify a solution so asthma due to prenatal nicotine exposure can be prevented.
INTRODUCTION
In the United States, approximately 25 million people are currently diagnosed with asthma [1]. Asthma is a respiratory condition characterized by difficulty breathing due to narrowing airways, caused by inflammation and excess mucus production. This inflammatory response is often triggered by viruses or air-borne allergens. Researchers are currently investigating the underlying immune mechanisms that cause the intense inflammatory response, which is often more intense when someone has been subjected to risk factors such as prenatal nicotine exposure. Since there is currently no cure for asthma, research about the underlying mechanisms of the inflammatory response is vital so that asthma can be prevented rather than simply managed.
Researchers have studied prenatal nicotine exposure and its effects on offspring for decades, focusing on human subjects who smoked while pregnant. Over the past thirty years, there has been a shift toward using animal trials to investigate the mechanisms associated with the risk factors for asthma.
The primary model in asthma research in mice is the house dust mite (HDM) model. The HDM model involves exposing one group of pregnant mice to tobacco smoke-infused air and another group of pregnant mice to filtered air. The offspring of both groups are exposed to house dust mites– a common allergen– and their inflammatory immune response is examined. There are variations to the model, such as exposing the fathers to nicotine prior to mating or exposing the female mice to nicotine prior to or during pregnancy.
Current literature cites three main factors that contribute to an increased risk of asthma: nicotine smoke exposure alone, placental damage induced by nicotine, and epigenetic alterations induced by nicotine. Nicotine passes from the mother’s blood to the fetus through the umbilical cord during pregnancy. Nicotine can also damage the placenta through vasoconstriction of blood vessels and alter the fetus’ epigenetic markers through DNA methylation.
The purpose of this literature review is to examine precisely how prenatal nicotine exposure increases the risk of asthma, first in experimental data using the HDM model and then in experimental & survey data regarding humans.
Prenatal Nicotine Smoke Exposure
In 2015, Eyring et al proposed that nicotine use in pregnant women increased the risk of asthma in offspring through epigenetic alterations [2]. Eyring et al exposed one group of female mice to tobacco infused smoke (ETS) for five weeks and mated them to male mice and examined the offspring. The pregnant female mice were then exposed to ETS until they gave birth. There was also a control group of female mice exposed to filtered air and mated to male mice. The offspring of the ETS exposed group did display an increased inflammatory response when exposed to house dust mites compared to the control group. However, the level of DNA expression of both groups were not statistically different. Thus, Eyring et al. came to the conclusion that prenatal nicotine exposure can cause an increased risk of asthma in offspring, but was unable to identify the mechanism through which prenatal ETS causes an increased inflammatory response [2]. It is possible that the Bisulfite sequencing equipment at the time of Eyring et al.’s study was not sensitive enough to detect the difference in methylation that newer studies observed.
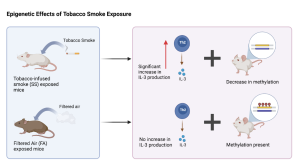
Figure 1. Expression levels of IL-5 (Th2 cytokine producing protein) are the same for the CS (ETS exposed group) and FA (filtered air group) mice when exposed to house dust mites (HDM). This indicated that the gene expression levels were not affected by ETS.
A three-generation survey study on human subjects found a correlation between maternal smoking and the increased risk of asthma in offspring, as well as a correlation between grandmothers smoking during pregnancy and their grandchildren having an increased risk of asthma, regardless of the intermediate generation’s smoking habits [3]. The researchers also found a correlation between paternal smoking and an increased risk of asthma in the offspring [3]. They have hypothesized that paternal smoking causes altered microRNA (miRNA) in the sperm. MiRNA is a nucleic acid that regulates expression of genes. During fertilization, this altered miRNA can change the gene expression of the progeny, increasing the risk of asthma in the offspring. The conclusion of this study is that maternal, paternal, and grandmaternal nicotine exposure is correlated with an increased risk of asthma in offspring. The researchers also proposed epigenetic alteration as the mechanism of increased asthma risk, but due to the nature of the study, they were unable to confirm this hypothesis [3].
Placental Damage
A survey of mothers who smoked and mothers who did not smoke by Zacharasiewicz et al. concluded that prenatal exposure to nicotine causes placental damage by decreasing nutrient delivery to the fetus [4]. Prenatal nicotine exposure decreases alveolar surface area, thereby decreasing the tidal volume of fetal lungs after birth [5]. Tidal volume is the amount of air that enters the lungs per breath. A decreased tidal volume results in less oxygen entering the body under standard conditions and a vastly reduced amount of oxygen entering the body when exposed to an allergen. Placental damage also results in the increased aging of the fetus’ lungs as pulmonary cells perform less glycogenolysis and glycolysis, causing cells to die prematurely [6]. The premature death of lung cells means the lungs are weaker, unable to exchange a normal amount of oxygen, and therefore more prone to intense allergic reactions.
Similarly, a study by Cahill et al. using the HDM mice model found that inhaling nicotine causes vasoconstriction– the narrowing of blood vessels– in the mother, resulting in less oxygen and nutrients delivered to the fetus [7]. They also found that placental HSD2 (a crucial enzyme in fetal development) is decreased when pregnant mothers are exposed to nicotine. Cahill et al also observed placental damage from nicotine use which resulted in decreased birth weights and lung size in fetuses [7]. Decreased lung size leads to intense asthmatic episodes because the airways are smaller and narrower than the airways of an individual not exposed to nicotine prenatally. Ultimately, Zacharasiewicz and Cahill came to the same conclusion that nicotine consumption or exposure in pregnant women increases the risk of asthma in their offspring by negatively affecting the offspring’s lungs [4,7].
Epigenetic Alteration
Researchers agree that DNA methylation is the one of the mechanisms leading to an increased asthma risk [8]. DNA methylation, the primary form of epigenetic alteration that occurs when a fetus is exposed to nicotine, is a chemical reaction where a methyl (-CH3) group is added to a cytosine base. This methyl group prevents transcription factors from binding to DNA and recruiting repression proteins, resulting in underexpressed genes, which in this case is a disproportionate inflammatory response. However, there is disagreement among researchers about which genes are being alternatively methylated. Christensen et al. conducted an HDM mouse study and found that methylation of genes which produce and regulate Th2 cytokines was decreased in the offspring of mothers exposed to ETS [9]. Cytokines are small proteins that regulate the immune response; Th2 cells produce cytokines that encourage inflammation. Thus the increased expression of Th2 intensifies the inflammatory response that occurs in response to the asthma trigger of house dust mites. Christensen et al. found that Th1 cytokine levels remained constant and methylation was unaffected [9].
Conversely, Singh et al. found that Th1 cytokine levels decreased due to hypermethylation [10]. Singh et al. did also find that Th2 cytokine levels increased due to hypomethylation, which concurs with the findings of Christensen et al [9-10].
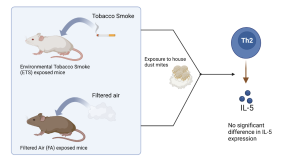
Figure 2. Expression levels of IL-3 (Th2 producing gene) in the groups that were exposed to tobacco infused smoke (SS) or filtered air (FA). There is a statistically significant increase in expression in the SS group indicating a decrease in methylation.
Christensen et al. exposed pregnant female mice to either tobacco smoke-infused air or filtered air and then examined the offspring [9]. Singh et al. exposed both male and female mice to tobacco smoke-infused air or filtered air prior to mating and then examined the offspring [10]. This variation in experimental methods could contribute to the difference seen in the methylation of Th1 cytokine-producing genes. However, both researchers concluded that the nicotine-induced DNA methylation levels changed in genes that produced inflammatory responses to allergens [9-10].
Zakarya et al. found that DNA methylation levels were altered in genes associated with fetal growth and nicotine detoxification [11]. This review examined epigenome-wide association studies (EWAS) on patients suffering from asthma whose mothers smoked or vaped during pregnancy. These studies showed increased methylation in placental, whole blood, and fetal lung genes [12]. These results differed from the research done by Singh et al. and Christensen et al. both in the affected genes and the way that methylation was altered [9-10]. The difference in results can be attributed to the difference between mice and humans as well as the variation in experimental design. Christensen and Singh used the HDM model on mice and controlled the levels of nicotine the mice were exposed to [9-10]. Zakarya et al. used data from children of women who reported smoking during pregnancy [11]. The levels of nicotine that the subjects were exposed to was not controlled and varied greatly. These differences between the studied species and experimental design could explain the different conclusions that the researchers drew.
CONCLUSION
There is not a simple answer about the mechanism by which nicotine use during pregnancy increases the risk of asthma in offspring. However, both epigenetic alterations and placental damage due to nicotine exposure play a role in increased asthma risk.
Research citing nicotine-induced epigenetic alteration as the main cause of the increased risk of asthma identifies various genes being altered by DNA methylation. The HDM studies cited in this review conclude that genes producing cytokines had a decrease in methylation, while a study using human subjects concluded that genes involving fetal growth and nicotine detoxification had an increase in methylation. Further research should determine which altered genes are increasing the risk of asthma so that methylation can be induced or repressed in those genes as a preventive measure for asthma. Further research should also focus on which aspect of nicotine-induced placental damage is the biggest factor in the increased risk of asthma so that a solution can be found to address that aspect.
Future research studies should continue to investigate the two presented mechanisms and identify the factors that are increasing the risk of asthma so that nicotine-induced asthma can be prevented in future generations.
