
Ophiocordyceps: How the Zombie Ant Fungus Manipulates Host Behavior
Abstract
Several parasites have the capacity to influence their host’s behavior. One example is Ophiocordyceps, a parasitic genus of fungi that infects ants of the Camponotus genus. The fungus hijacks the ant’s body, manipulating the ant’s behaviors to further its own propagation. Prominent abnormal behaviors this fungus induces in ants include disrupted circadian rhythms and foraging habits, summiting, and mandibular contraction. These behaviors—known as extended phenotypes—and the factors that determine when, where, why, and how they occur are the subjects of this review. Most of the literature covered in this review was limited to the BioSIS database, with priority placed on primary research papers published within the last five years. Those papers include genomic analyses, field surveys, and various forms of microscopy, which grant insight into how Ophiocordyceps functions. For example, observations of fungal hyphae within ant bodies indicate this fungus manipulates behavior without directly invading the brain. Additionally, scientists found that light levels and humidity affect expression of extended phenotypes. However, many of these unusual behaviors vary by Ophiocordyceps and Camponotus species, so further research is needed. Dissecting the nuances of this host-parasite relationship contributes to the overall understanding of parasites. Although this particular fungus only infects ants, there are parasites that affect other insects and mammals in remarkably similar ways to those of Ophiocordyceps. As a result, these ants and their fungal parasites serve as model organisms for entomologists and parasitologists.
Keywords: Behavioral manipulation, Biting behavior, Extended phenotype, Ophiocordyceps
Introduction
Many people have played the zombie-apocalypse video game The Last of Us, or watched the hit TV series by the same name. In this series, a fungus infects humans and takes control of their minds, mutating them into putrid monsters. That fictional fungus was inspired by Ophiocordyceps, a genus of parasitic fungi that infects ants of the Camponotus genus, commonly known as carpenter ants. Ophiocordyceps is noteworthy in that it influences ant behavior to further its own propagation. It causes ants to climb up plants and then clamp on with their mandibles, where they eventually die [1]. The mechanism of action of Ophiocordyceps involves a variety of factors, including genetics, muscular dynamics, light, and humidity. Additionally, recent studies have found that, unlike other parasites that alter behavior, the fungal cells leave the ant’s brain intact. Instead, they infect the muscles of ant mandibles, causing them to clamp shut [2].

Even though this fungus cannot affect humans, other parasites can. Studying the processes that underlie Ophiocordyceps infection can help us enhance our understanding of human parasites and other pathogens. This review will compile known factors and mechanisms involved in Ophiocordyceps’ manipulation of its host, from initial infection to death. The main topics to be discussed are behavioral changes in foraging, summiting, and mandible contraction, including relevant external stimuli.
Changes in Circadian Rhythm and Foraging
Using modern genetic sequencing and analysis technologies, researchers were able to investigate the genetic basis of the behavioral manipulations observed when Ophiocordyceps infects carpenter ants. They assembled the genomes of Ophiocordyceps camponoti-floridani and Camponotus floridanus, and compared that data to the genomes of Ophiocordyceps kimflemingiae and Camponotus castaneus, using weighted gene coexpression network analyses (WGCNA analyses). These computational tools show how sets of genes called modules are coexpressed. By performing this analysis on genomic data from both the ant and the fungus, they were able to find modules of genes that are upregulated or downregulated together. From this observed coexpression, the researchers identified genes involved in behavior manipulation, including genes related to ant circadian rhythms and foraging behavior, that may be influenced by the fungi. The fungus manipulates host genes by secreting secondary metabolites, such as mycotoxins, during infection [3]. This is important to understand, as other parasites may affect their hosts by modifying expression of the same genes or analogous ones.
Based on these findings, analysts further investigated how ant circadian rhythms are affected by Ophiocordyceps. In their literature review, De Bekker & Das performed their own WGCNA analysis. Some of the modules they looked at affect behaviors that peak during daytime/nighttime, which depend on the social caste the ant belongs to [4]. Two main castes are nurses, which stay at home to raise offspring, and foragers, which venture out to find food. The genes that affect which caste an ant belongs to at a given time are called behavioral plasticity genes. The researchers found that these genes are ideal targets for the fungus because they directly control host behavior. By influencing these genes, the fungus implements what are known as extended phenotypes. These are phenotypes of one organism that are influenced by genes from another organism’s cells [5]. In this case, the altered circadian rhythm and caste-related behaviors exhibited by infected ants are considered extended phenotypes.
Most studies involving Ophiocordyceps and Camponotus focus on extended phenotypes occurring immediately before host death, so in 2021, researchers sought to determine if any changes in ant behavior arise earlier on in their infection. Specifically, they looked into the possibility of irregular foraging behavior. They set up two mazes in a lab: one for infected ants and one for healthy ants, which served as the control. They placed food in these mazes, for the purpose of observing how route optimization may be affected by Ophiocordyceps. Not only were the infected ants less efficient with their routes, they also left their nests at abnormal times. Healthy ants, for example, do not leave their nest during the daytime, but the infected ants did [6]. This serves as further evidence that host circadian rhythm is disrupted by Ophiocordyceps. By manipulating the ants’ internal clocks in this way, the fungus increases the probability of placing its host in proper conditions for summiting and fruiting.
Summiting
The term “summiting" describes the final vertical ascent of the ant. Ophiocordyceps forces it to climb up vegetation to a certain height, which varies by fungus and ant species. The ant will die there. There are a number of factors at work during summiting, including light levels, corpse permanence, and humidity. To observe exactly how light affects this summiting behavior, scientists ventured into the Amazon rainforest to survey regions called “ant graveyards.” These are sections of rainforest populated with ants that died due to Ophiocordyceps. After setting up screens to manipulate the levels of available light around these areas, they observed differences in the number of dead ants. Shaded areas had significantly fewer infected corpses than the control. Ant cadaver height also varied with light levels; they found dead ants higher up in the shade than in the sun. Lastly, they found fewer fruiting bodies in the shade [7]. All of this indicates that the fungus fruits under higher levels of light, and manipulates its host to move up to conditions ideal for its fruiting. Inducing summiting behavior is not unique to Ophiocordyceps. For example, baculoviruses infect caterpillars and drive them up plant stems, where they will die and “rain down” the virus onto other unsuspecting hosts below [8].
Based on this study and previous observations of dead ants, it is clear that summiting is a common strategy Ophiocordyceps employs to better disperse its spores. However, it remains unclear whether a higher summit point always corresponds to better spore dispersal. Andriolli et. al continued their previous research and conducted another survey of the Amazon rainforest to see if this summiting strategy is universally successful across three species of the Ophiocordyceps genus [9]. They used a metric called “corpse permanence,” which describes how long the corpses persist in the environment before getting eaten by predators or otherwise destroyed. The higher a corpse is, the more likely it is to be found by predators like birds. These predators are a threat to the fungus, as it cannot disperse its spores if it gets eaten directly after forming a fruiting body. They found that some fungal species have longer corpse permanence up high, while other species last longer towards the forest floor. Overall, higher summiting does not always equate to success; ideal summit height depends on fungal species.
While light levels affect summiting, humidity must also be acknowledged. Ophiocordyceps is a tropical genus, and thus lives in areas of high air humidity along the equator. Researchers counted ants of various Camponotus species killed by these fungi in Brazil over the course of 14 months to measure how fluctuating air humidity levels over time and space affect fungal prevalence. They observed a noticeable pattern of infection due to humidity within one of the species they documented, Ophiocordyceps unilateralis [10]. They found a positive correlation between the number of infected corpses and temporal air humidity. Fungi prefer moist conditions, so it follows that more infected corpses are found during times of higher humidity. However, they observed that the number of dead Camponotus senex ants did not correlate with air humidity. Instead, dead ants of that species were more commonly found in drier areas where the groundwater was deeper underground, further away from the surface. C. senex ants were also found higher up in the canopy, even further from groundwater [10]. This was an unexpected result, as small animals such as insects are prone to desiccation (drying out), which would be detrimental to a parasitic fungus that needs water to survive. Further research is needed to determine why exactly this occurs. On the whole, these results suggest that the summiting extended phenotype not only varies by Ophiocordyceps species, but also by Camponotus species.
Mandible Contraction
Once the ant has reached its summit, it will irreversibly clamp down on the plant with its mandibles. Experts in the field refer to this behavior as mandibular contraction. The exact methods the fungus utilizes to accomplish this were only recently discovered. Looking at ant corpses, one can easily tell that the fungus entered the head, because the fungal fruiting bodies sprout from the base of the head. However, that alone is not enough information to deduce how the fungus affects the mandibles. Closer examination of host morphology is necessary.
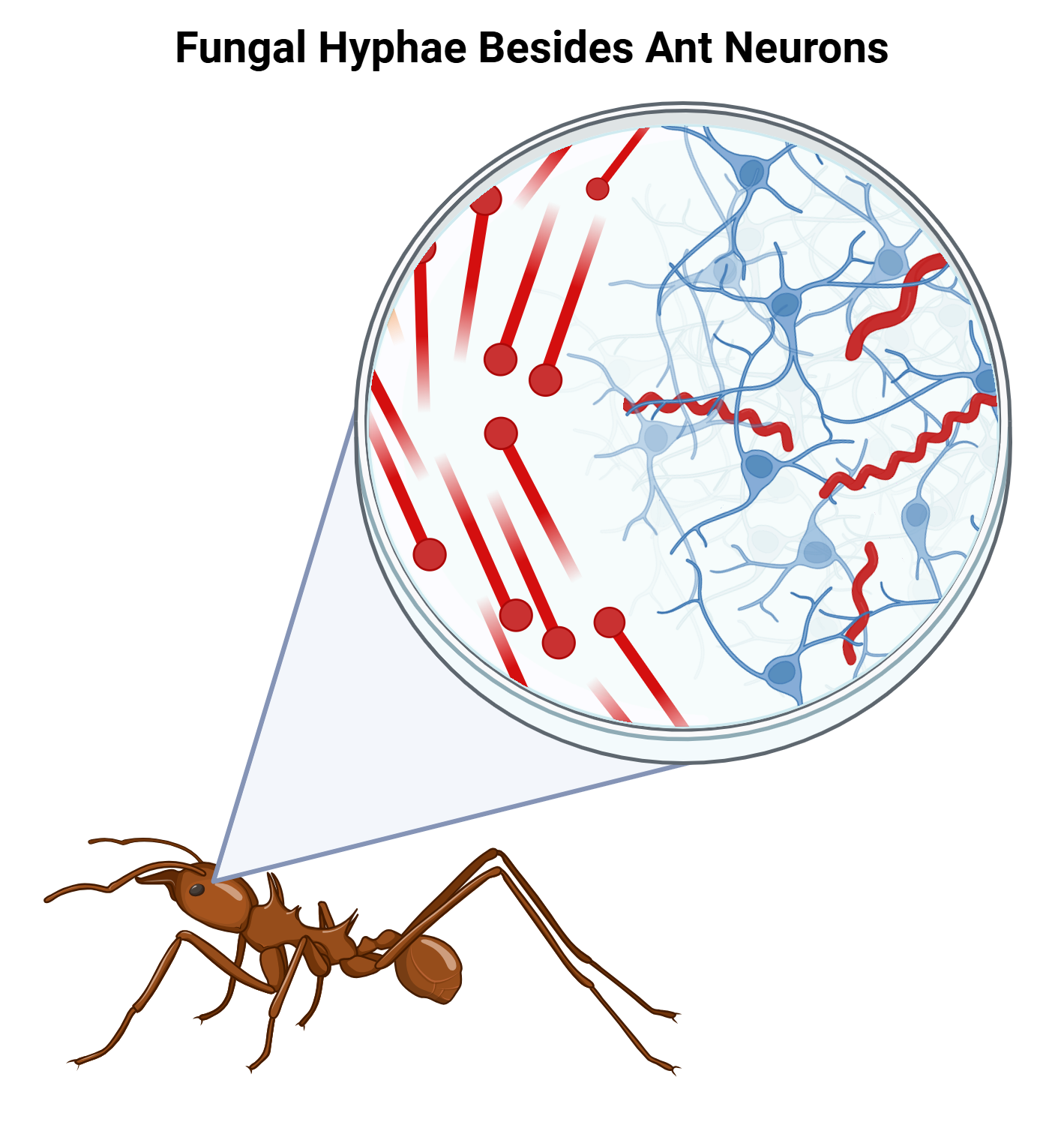
In 2017, Fredericksen et. al. utilized fluorescence confocal microscopy to confirm exactly where Ophiocordyceps is found within the ant. Specifically, they took interest in the brain, as that is the main target of many other parasites affecting behavior, such as Toxoplasma gondii, a parasite that invades the brains of mice and causes them to be attracted to the smell of cat urine [11]. Using ants infected with Ophiocordyceps in a lab, they colored the ant neuronal synapses fluorescent green with antibodies and fungal cells fluorescent red with a stain. Their counterstaining technique revealed that fungal hyphae do not enter the brain. While there were red cells found in the brain, these cells were determined to be tracheae, which are small tubes that deliver oxygen throughout the body, rather than fungal cells [2]. They likely observed this because the stain they used, calcofluor white anti-chitin stain, binds to chitin. While there is chitin in the fungal cell walls, ant tracheae are also lined with chitin, and the hyphae and tracheae are too different in shape to be confused with one another. This was a significant discovery, demonstrating that physically invading the brain may not be required for parasites to influence host behavior.
Observing that this extended phenotype likely does not originate from the brain, researchers moved onto the mandibular muscles. Fredericksen et. al. proceeded to use scanning electron microscopy (SEM) to get clear images of the ant musculature and how it was affected by the fungus. They found many fungal hyphal cells among the mandibular muscle cells, accompanied by extensive muscle damage [2]. This indicates that the fungus directly affects mandibular contraction. However, it remained unclear how the fungus still retained control of the muscles after causing so much damage.
Two years later, Mangold et. al. sought to fill that knowledge gap. Continuing Fredericksen’s line of research, Mangold’s team worked to understand the specific interactions between ant muscle cells and fungal hyphal cells. They used SEM and TEM (transmission electron microscopy) to look more closely at the fungal cells and ant musculature. Their findings included z-line swelling, sarcomere shortening, and sarcolemma blebbing, all of which are forms of muscle damage that indicate a state of hypercontraction rather than atrophy [1]. This would explain how the mandibles remain permanently clamped around substrate, even after death.
Conclusion
Ultimately, the extended phenotypes of irregular foraging, summiting, and mandibular contraction expressed by Camponotus ants due to Ophiocordyceps infection are diverse in manifestation due to a variety of factors. They all arise from highly regulated coexpression of fungal and ant genes. They vary by light level, humidity level, individual fungal species, and individual ant species. Further avenues of research include analyses of these interspecies differences, as some of them are counterintuitive to previously established norms. Comprehending this relationship to the fullest possible extent may lead to further insight into parasitic organisms with more direct influence over humans, as many of these manipulated behaviors are expressed in other parasite-host interactions.

About the Author: Lyndsey Rowan
Lyndsey Rowan is a third-year Molecular & Medical Microbiology Major and is working towards a minor in Medical-Veterinary Entomology. She chose her major because she enjoys observing the unseen world of microbes and learning about their potential uses for society. After undergrad, she wants to go to graduate school for microbiology or infectious disease. She initially wrote this piece for her UWP 102B class: Writing in Bio Sci, so its target audience is college undergraduates with a background in biology. Her interests include microbiology and entomology, and reading about the zombie ant fungus was a fun way to get a dose of both fields. She first learned of Ophiocordyceps from a nature documentary, then more people began discussing it when The Last of Us got released. That made her realize she never knew exactly how the fungus worked. She heard bits and pieces of what the fungus does to its host, but knew nothing about the mechanisms and factors involved. She figured many other students were in the same boat, so she wrote this review for those of us who are interested in learning more.
References
Mangold, C. A., Ishler, M. J., Loreto, R. G., Hazen, M. L., & Hughes, D. P. (2019). Zombie ant death grip due to hypercontracted mandibular muscles. J. Exp. Biol, 222(14), jeb200683. https://doi.org/10.1242/jeb.200683
Fredericksen, M. A., Zhang, Y., Hazen, M. L., Loreto, R. G., Mangold, C. A., Chen, D. Z., & Hughes, D. P. (2017). Three-dimensional visualization and a deep-learning model reveal complex fungal parasite networks in behaviorally manipulated ants. PNAS, 114(47), 12590–12595. https://doi.org/10.1073/pnas.1711673114
Will, I., Das, B., Trinh, T., Brachmann, A., Ohm, R. A., & de Bekker, C. (2020). Genetic Underpinnings of Host Manipulation by Ophiocordyceps as Revealed by Comparative Transcriptomics. G3 (Bethesda), 10(7), 2275–2296. https://doi.org/10.1534/g3.120.401290
De Bekker, C., & Das, B. (2022). Hijacking time: How Ophiocordyceps fungi could be using ant host clocks to manipulate behavior. Parasite Immunol, 44(3), e12909. https://doi.org/10.1111/pim.12909
Valentino, S. I., & Greenspan, N. S. (2019). Extended phenotype in evolutionary medicine. Evol. Med. Public Health, 2019(1), 48–49. https://doi.org/10.1093/emph/eoz009
Trinh, T., Ouellette, R., & de Bekker, C. (2021). Getting lost: The fungal hijacking of ant foraging behaviour in space and time. Anim. Behav, 181, 165–184. https://doi.org/10.1016/j.anbehav.2021.09.003
Andriolli, F. S., Ishikawa, N. K., Vargas-Isla, R., Cabral, T. S., de Bekker, C., & Baccaro, F. B. (2019). Do zombie ant fungi turn their hosts into light seekers? Behav. Ecol, 30(3), 609–616. https://doi.org/10.1093/beheco/ary198
Katsuma, S., Koyano, Y., Kang, W., Kokusho, R., Kamita, S. G., & Shimada, T. (2012). The Baculovirus Uses a Captured Host Phosphatase to Induce Enhanced Locomotory Activity in Host Caterpillars. PLoS Pathog, 8(4), e1002644. https://doi.org/10.1371/journal.ppat.1002644
Andriolli, F. S., Cardoso Neto, J. A., De Morais, J. W., & Baccaro, F. B. (2024). With the dead under the mat: The zombie ant extended phenotype under a new perspective. Sci. Nat, 111(4), 33. https://doi.org/10.1007/s00114-024-01920-w
Cardoso Neto, J. A., Leal, L. C., & Baccaro, F. B. (2019). Temporal and spatial gradients of humidity shape the occurrence and the behavioral manipulation of ants infected by entomopathogenic fungi in Central Amazon. Fungal Ecol, 42, 100871. https://doi.org/10.1016/j.funeco.2019.100871
Gatkowska, J., Wieczorek, M., Dziadek, B., Dzitko, K., & Dlugonska, H. (2012). Behavioral changes in mice caused by Toxoplasma gondii invasion of brain. Parasitol. Res, 111(1), 53–58. https://doi.org/10.1007/s00436-011-2800-y
