
A Prospective Outlook on Tackling Acute Kidney Injury: from Diagnosis to Treatment
Introduction
Acute kidney injury (AKI) is a common complication found in hospitals and the third leading cause of death among trauma patients [3]. Acute kidney injury (AKI) is characterized by the sudden loss of kidney function. Consequently, the lack of a filtering system in the body leads to systematic damage. The prevalence of kidney-related deaths can be attributed to all the potential causes of AKI, such as renal hypoperfusion, nephrotoxic exposure, sepsis, and major surgery [7]. Research shows that these causes share conditions such as inflammation and oxidative stress [5, 8]. The wide variety of AKI causes can be attributed to a few pathophysiologies, and studies are exploring medications that target these. These medications can potentially provide a solution and treat multiple AKI sources at once.
In conjunction with improving treatments, there is also a need to improve AKI diagnosis. For instance, serum creatinine is a biomarker that is commonly measured to indicate signs of AKI in hospitals, but recent studies have highlighted its limitations [6, 7]. A suboptimal indicator can delay treatment until late-stage complications occur. This review will explore developments of new biomarkers and antioxidant medications that address these concerns.
The Need for New Development of Biomarkers to Diagnose Acute Kidney Injury
The Insufficiencies of Serum Creatinine
Recent research has highlighted key limitations of serum creatinine as an indicator due to its delayed and inconsistent detection abilities. During AKI, damaged kidney cells release different chemicals into the bloodstream. Researchers can measure the level of these chemicals to determine the degree of damage. One of these chemicals is creatinine, a waste product that the kidney usually removes. Creatinine concentrations are prone to vary significantly during different medical treatments and conditions, such as renal replacement therapy (RRT) and liver cirrhosis. The study by Groote et al. [9] examined serum creatinine and other biomarkers during RRT, which is a rescue therapy that about 5-13% of AKI patients undergo until they restore normal kidney function. Groote et al. observed that with creatinine, there was no set creatinine concentration that indicated whether one had a damaged kidney. As a result, experts had to make subjective judgments when using creatinine. Additionally, creatinine levels were elevated during RRT despite the lack of kidney damage [9]. Similarly, research done by Yang et. al [11] further highlights the limitations of serum creatinine by studying patients with liver cirrhosis, another condition that leads to AKI. Compared to other biomarkers, Yang et al. reported that serum creatinine detected AKI in its later stages [11]. At that point, kidney damage already progressed to more severe levels. Furthermore, serum creatinine levels are not consistent enough to reveal the presence of AKI because they are affected by external factors such as diet, age, and gender [11]. These studies showcase the shortcomings of creatinine in two situations: RRT and liver cirrhosis. Despite these limitations, Yang et al. [11] acknowledged that creatinine testing is cheap and easy to perform. Creatinine detection can still be a valid option in a region with limited resources and money. As a long-term solution, however, the study results call for new diagnostic alternatives for AKI.
New Potential Biomarkers
Neutrophil gelatinase-associated lipocalin (NGAL) and proenkephalin, two new potential biomarkers, are chemicals that are released in damaged renal cells. Yang et al. [11] proposed measuring NGAL levels as a promising biomarker. NGAL is a protein that is released by renal cells when they depart from homeostasis. The researchers tested three biomarkers in total, and NGAL was the most effective at detecting AKI. NGAL was more sensitive to AKI at earlier stages, compared to other biomarkers [11]. The researchers explained that at earlier stages of AKI, the kidney is able to maintain normal function at first despite damage, preventing the rise of creatinine. However, NGAL levels can still rise because damaged renal tissues promote its expression, regardless of the compensatory mechanisms in place [11]. While NGAL appears to be effective in diagnosing AKI, proenkephalin seems to be useful in measuring kidney function after AKI has already been established. Groote et al’s study sought to use proenkephalin as a biomarker for underlying kidney function [9]. They assessed kidney function to determine when AKI patients undergoing RRT could be released. Proenkephalin showed promising results with high success rates. Groote et al. [9] compared proenkephalin to NGAL, and indicated that NGAL was not as effective as proenkephalin to determine kidney function. They observed that differences detected by proenkephalin were not reflected by NGAL. However, these results were in the context of patients who already had AKI; there was no discussion about using proenkephalin to diagnose it [9]. Thus, more research needs to be done on whether NGAL or proenkephalin should be used to diagnose AKI at early stages. As more research is done, health professionals will be able to determine the optimal biomarkers to use in any setting.
Medications that Target Oxidative Stress are Promising
Alpha-2-Adrenergic Agonists

Medications that alleviate oxidative stress can treat multiple AKI conditions by acting on alpha-2-adrenergic receptors. Some AKI-causing conditions are sepsis, recovery after surgery, and diabetes. Kalakeche et al.’s study highlights the role of oxidative damage on the kidney during sepsis [4]. The researchers used fluorescence to track the pathway of endotoxins during infection. They then observed levels of endotoxins in the kidney using photon imaging. They found that the kidney takes in significant amounts of endotoxins, along with an increased amount of oxidative species. Oxidative species can damage other compounds through the release of toxic compounds via oxidation. These results displayed that oxidative stress is a primary mechanism of sepsis-driven AKI [4]. Clinical trials for medications that treat oxidative stress support these results: by reducing oxidative stress, the medications successfully treated AKI. One study tested the effects of dexmedetomidine (DEX), an antioxidant, to treat AKI during sepsis [10]. Feng et al. determined that DEX’s antioxidant effect centers around its role as an agonist for alpha-2-adrenergic receptors and imidazoline receptors [10]. Mice were induced with AKI via sepsis, and treatment with DEX alleviated AKI [10]. These results strengthen Kalakeche et al.’s conclusion that oxidative stress and renal impairment are directly connected. Another study by Tang et al. [8] tested the effects of DEX in patients who developed AKI after heart valve replacement surgery, in which approximately 50% of patients who underwent that surgery developed AKI. Clinical trials showed that AKI patients who received DEX had lower levels of CTnI, CK-MB, and MDA, which are indicators of impaired renal function [8]. The studies from Feng et al. and Kalakeche show how the same medication can treat AKI in two different contexts because of their similar pathophysiology. While sepsis and post-surgery are only two examples among many that can cause AKI, determining the overlapping mechanisms and targeting these overlaps can make treating AKI more efficient.
Plant-Based Remedy
Certain plants such as curcumin, a type of spice and medicinal herb, have antioxidant effects that can also treat AKI. Machado et al. [6] studied the use of curcumin in another context of AKI: diabetes. Diabetes can damage the kidneys through oxidative stress because high glucose levels lead to sustained glucose metabolism. As a result, this produces reactive oxygen species, which are primary agents for oxidative stress. Over the course of a month, diabetic mice who received curcumin had reduced creatinine and increased inulin clearance, both of which point to improved renal function [6]. These results are similar to the results of DEX [8]. More research should be done to compare the potential use of DEX versus curcumin. As a natural plant, dietary incorporation of curcumin to treat AKI can be cost-effective and accessible. While DEX is considerably more expensive, DEX offers other benefits including sedative effects and a low half-life [8]. From a cost standpoint, curcumin seems to be more practical, but DEX may be more effective overall in treating AKI. The studies from Feng et al. [10] and Tang et al. [8] established that DEX can be used in multiple AKI conditions, but there haven’t been many studies to test curcumin with other AKI conditions.
Histone Deacetylase Inhibitors
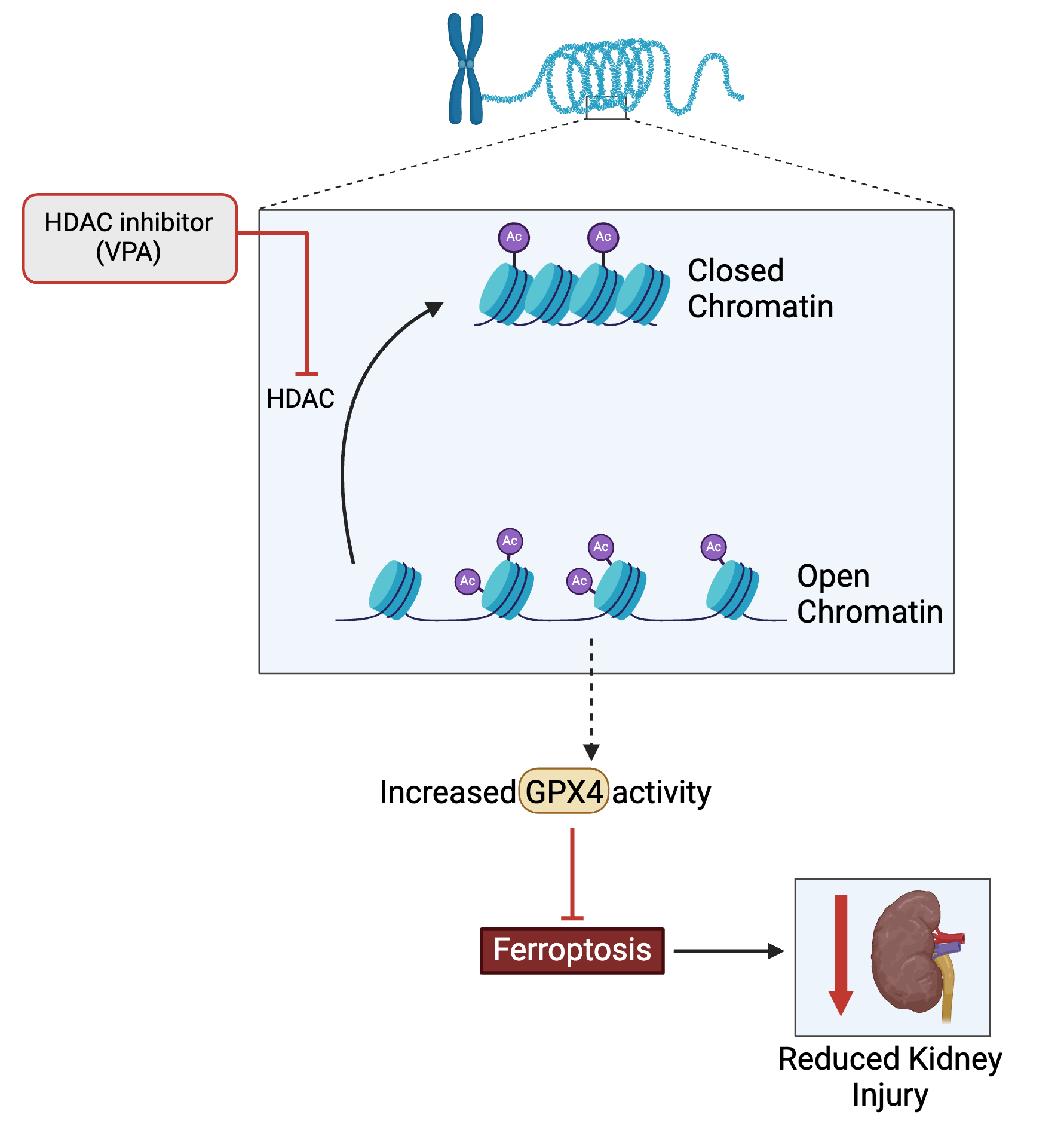
Epigenetics is a promising field of study to treat oxidative stress in AKI. Recent studies have applied valproic acid (VPA), a histone deacetylase inhibitor (HDAC), to treat several different AKI causes such as trauma and chemotherapy. One study by Biesterveld et al. examined valproic acid as a potential medication option for AKI caused by trauma and hemorrhage [1]. Swine were subjected to various forms of trauma and then treated with VPA. The researchers found that single-dose VPA treatments increased survival rates from 7% to 83%, along with improved renal function. While the researchers were able to conclude that VPA supports AKI, there was little insight into how it did so [1]. A more recent study by Li et al. provided more insight into this by proposing that VPA alleviated oxidative stress in AKI subjects through ferroptosis inhibition, which is a form of cell death that is associated with the production of reactive oxygen species [2, 5]. Li et al. [5] compared protein levels in cell cultures of mice who received a chemotherapy drug, cisplatin. The primary proteins of interest were GPX4 (an enzyme) and histone deacetylases, which are enzymes that remove acetyl groups from histones. These comparisons indicated that the GPX4 enzyme inhibited ferroptosis. The researchers also measured that increases in histone deacetylase inhibitors (HDAC) decreased levels of GPX4. Thus, the sequence of events that VPA induces is: inhibiting HDAC, which enables GPX4 activity, inhibiting ferroptosis and thus reducing kidney injury. The researchers analyzed GPX4’s genome sequence and determined that HDAC could control the gene expression of GPX4, giving further support to their proposed sequence of events [5]. These studies not only highlight the effectiveness of valproic acid but also how inhibiting HDAC can resolve multiple causes of AKI. Even so, there are still some concerns about VPA. Biesterveld et al.’s study had positive results because they used a single dose of VPA. However, they indicated that repeated usage of VPA aggravated oxidative stress and caused further renal damage [1]. Considering that HDAC inhibitors can treat injuries in various other organs, the aggravation may be attributed to another effect [1]. Given how new this development is, more research should be done to better understand HDAC.
Conclusion
The purpose of this paper was to show the recent development of new biomarkers and medications that seek to treat central AKI mechanisms. AKI diagnosis and treatment need more improvement. It is problematic that serum creatinine is the most widely used biomarker for AKI, given its nonoptimal sensitivity. From a medicinal standpoint, researchers still have more to learn about the exact mechanisms of AKI. As such, medicinal research is still at an early stage. Current research has identified the most prominent causes of AKI such as sepsis and hemorrhage–leading to strong evidence that oxidative stress is a primary mechanistic link among these causes. Treatments that target oxidative stress have been able to treat multiple causes of AKI, but they should be tested more before being used in a clinical setting. Future research should be conducted to determine more mechanisms that lead to AKI because that appears to be the limiting factor in developing medications. The discovery of more mechanisms will open up more treatment options for AKI. To expand past alleviating oxidative stress, medications that target renal inflammation may be a viable option, since that is another possible central mechanism of AKI.

About the Author: Andrew Tran
Andrew is a class of 2024 Biological Sciences major.
Author's Note
I wrote this piece during my UWP 102B class and really struggled finding a topic I was interested in. My topic changed about five times because each time I started writing, I discovered that I was not interested in the topic. After digging around for several nights, I stumbled upon acute kidney injury, which is a huge problem hospitals face today. I hadn’t heard much about it, but upon researching more I learned that much still needs to be done to tackle this issue. With this literature review, I hope that it gives another avenue for others to stumble upon and learn more about this condition, just like I did.
References
- Biesterveld BE, Siddiqui AZ, O'Connell RL, Remmer H, Williams AM, Shamshad A, Smith WM, Kemp MT, Wakam GK, Alam HB. Valproic Acid Protects Against Acute Kidney Injury in Hemorrhage and Trauma. J Surg Res. 2021 Oct. doi: https://10.1016/j.jss.2021.04.014.
- Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012 May 25. doi: 10.1016/j.cell.2012.03.042.
- Harrois, A., Soyer, B., Gauss, T. et al. Prevalence and risk factors for acute kidney injury among trauma patients: a multicenter cohort study. Crit Care 22, 344 (2018). doi: https://doi.org/10.1186/s13054-018-2265-9
- Kalakeche R, Hato T, Rhodes G, Dunn KW, El-Achkar TM, Plotkin Z, Sandoval RM, Dagher PC. Endotoxin uptake by S1 proximal tubular segment causes oxidative stress in the downstream S2 segment. J Am Soc Nephrol. 2011 Aug 22. doi: 10.1681/ASN.2011020203.
- Li Y, Li K, Zhao W, Wang H, Xue X, Chen X, Li W, Xu P, Wang K, Liu P, Tian X, Fu R. VPA improves ferroptosis in tubular epithelial cells after cisplatin-induced acute kidney injury. Front Pharmacol. 2023 Apr 19. doi: https://10.3389/fphar.2023.1147772.
- Machado DI, de Oliveira Silva E, Ventura S, Vattimo MFF. The Effect of Curcumin on Renal Ischemia/Reperfusion Injury in Diabetic Rats. Nutrients. 2022 Jul 7. doi: https://doi.org/10.3390/nu14142798.
- Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. 2019 Nov 23. doi: https://doi.org/10.1016/S0140-6736(19)32563-2.
- Tang C, Hu Y, Gao J, Jiang J, Shi S, Wang J, Geng Q, Liang X, Chai X. Dexmedetomidine pretreatment attenuates myocardial ischemia reperfusion induced acute kidney injury and endoplasmic reticulum stress in human and rat. Life Sci. 2020 Sep 15. doi: https://10.1016/j.lfs.2020.118004.
- von Groote T, Albert F, Meersch M, Koch R, Porschen C, Hartmann O, Bergmann D, Pickkers P, Zarbock A. Proenkephalin A 119-159 predicts early and successful liberation from renal replacement therapy in critically ill patients with acute kidney injury: a post hoc analysis of the ELAIN trial. Crit Care. 2022. doi: https://doi.org/10.1186/s13054-022-04217-4
- Xiujing Feng X, Guan W, Zhao Y, Wang C, Song M, Yao Y, Yang T, Fan H. Dexmedetomidine ameliorates lipopolysaccharide-induced acute kidney injury in rats by inhibiting inflammation and oxidative stress via the GSK-3β/Nrf2 signaling pathway. J Cell Physiol. 2019 Aug 23. doi: https://doi.org/10.1002/jcp.28539.
- Yang Y, Ge B, Liu Y, Feng J. The efficacy of biomarkers in the diagnosis of acute kidney injury secondary to liver cirrhosis. Medicine (Baltimore). 2021. doi: https://doi.org/10.1097/MD.0000000000025411.
