
The potential of ketogenic diets as an adjunctive cancer therapy
Introduction
Cancer is a complicated and heterogeneous disease, characterized by its diversity within a cancerous tumor or between different tumors in terms of their genetic, molecular, cellular, and functional characteristics. This diversity contributes to significant morbidity and mortality around the world. Current cancer treatments, such as surgery, chemotherapy, and radiation, are currently limited by factors such as tumor resistance, metastasis, and adverse side effects [1]. The critical need for novel therapeutic options to improve patient outcomes and survival rates has motivated the investigation of novel cancer management approaches. Integrating dietary treatments with chemotherapy and immunotherapy has emerged as a viable technique to improve treatment success in recent years [2, 3, 4]. One such example are ketogenic diets (KDs), which have garnered attention for their anti-tumor and anti-inflammatory characteristics among various dietary treatments [1]. This paper consolidates data on many forms of solid tumors such as pancreatic, lung, breast, head and neck cell carcinoma, and melanoma. These tumors frequently display resistance to conventional chemotherapy and immunotherapy treatments, underlining the need for novel treatments. This evaluation of the literature examines the effect of a ketogenic diet in combination with chemotherapy or immunotherapy on cancer patient survival rates. It also investigates metabolic changes and associated risks with these combined therapies.
Unique features of ketogenic diets

KDs, which are high in fat, moderate in protein, and have a very low carbohydrate content, were originally devised to treat refractory epilepsy [1]. KDs differ from other high-fat diets in their capacity to induce nutritional ketosis, a state in which the body adjusts to using fats and amino acids as the primary energy source and produces ketone bodies as byproducts [5,6]. Ketone bodies exhibit anti-inflammatory properties and have been shown to reduce production of pro-inflammatory markers such as IL-17 and IL-18 [7]. Consequently, metabolic changes associated with ketone bodies may have an influence on cancer treatment [6]. Cancer cells express higher amounts of oxidative stress - an imbalance between free radicals and antioxidants in the body - than normal cells. Free radicals under normal conditions help fight off pathogens, but excessive production can cause DNA, fatty tissue, and protein damage due to their high reactivity. By reducing the production of free radicals, ketone bodies may indirectly limit cancer cell proliferation and promote apoptosis, programmed cell death, of cancer cells [8,9].
The rationale for including ketogenic diets in therapy for solid tumors lies in the understanding that they have the potential to discriminately target cancer cells without targeting normal cells. This potentially happens because depriving cancer cells of glucose, their primary energy source through glycolysis, could hinder growth [10]. Glycolysis is an anaerobic process - does not require oxygen - and produces pyruvate as a byproduct. Under normal circumstances, the presence of oxygen would allow the pyruvate to go through the citric acid cycle and eventually produce ATP through oxidative phosphorylation. However, under low oxygen conditions, the pyruvate would instead undergo fermentation to recycle products that can be reused for glycolysis. The presence of oxygen allows for more efficient production of ATP since glycolysis only produces a net ATP amount of 2 per glucose molecule compared to approximately 30 during oxidative phosphorylation. Surprisingly, cancer cells undergo aerobic glycolysis - also known as the Warburg effect - where even in the presence of oxygen, they will more readily use glycolysis to produce ATP [11]. Since glycolysis is not as efficient in producing ATP compared to oxidative phosphorylation, cancer cells have a higher dependency on glucose than normal cells. Hence, by creating an environment where glucose is scarce due to their limited carbohydrate content, KDs have the potential to starve cancer cells. On the other hand, normal cells that are highly dependent on glucose to function, such as those in the brain, can compensate for the lack of glucose by using ketone bodies as an alternative energy source. While it has been understood that cancer cells don’t have the capacity to use ketone bodies as an energy source, this process is not well understood. [12].

How ketogenic diets could benefit current treatments against solid tumors
Solid tumors often contain hypoxic regions with limited oxygenation, making them resistant to oxygen-dependent chemotherapy drugs [13]. Furthermore, solid tumors create an immunosuppressive microenvironment. The characteristics of this microenvironment vary depending on the type and stage of cancer. Typically, it consists of cancer cells, stromal cells like fibroblasts that interact with cancer, immune cells, the extracellular matrix providing structural support, blood vessels, and signaling molecules. Additionally, there are immunosuppressive factors that impede immune cell activity, resulting in low levels of tumor-infiltrating lymphocytes [14].
Tumor cells can evolve strategies to evade immune recognition and develop resistance to immunotherapy agents. One common mechanism of resistance involves mutations or alterations in the genes or pathways targeted by immunotherapies, making them less effective over time [15]. Tumors may also upregulate alternative immune checkpoint proteins or employ other immunosuppressive strategies, further complicating the treatment landscape [16].
The ketogenic diet's potential benefits in cancer treatment also stems from inducing a metabolic shift that alters the tumor microenvironment. Following a KD may lead cancer cells to enter nutritional ketosis, increasing their vulnerability to select chemotherapy drugs treatments [17]. This heightened therapy sensitivity has the potential to enhance treatment effectiveness when combined with a ketogenic diet.
Effect of a ketogenic diet coupled with chemotherapy vs chemotherapy alone
Overall survival
The addition of a ketogenic diet to chemotherapy has shown improved cancer survival rates compared to chemotherapy alone [18,19]. Zahra et al. conducted two Phase 1 clinical trials. These trials involved patients with locally advanced lung or pancreatic cancer. Locally advanced cancers are tumors that have grown beyond their site of origin and invaded nearby tissues but have not spread to distant body parts [12]. The patients were assigned to receive either chemotherapy alone or chemotherapy in combination with a ketogenic diet. The group receiving the combined treatment demonstrated enhanced treatment responses, including a higher rate of tumor shrinkage and disease control. Moreover, patients following the combined treatment had better overall survival rates than those receiving chemotherapy alone. However, it is important to note that this study was limited to Phase 1 trials, which primarily focus on safety evaluation rather than long-term survival outcomes [18]. Further research is necessary to confirm the long-term efficacy of combined treatment.
A study conducted by Cortez et al. further supports the positive outcome of combining chemotherapy with a ketogenic diet compared to chemotherapy alone [19]. Researchers utilized a mouse model of pancreatic cancer (KPC mice) to evaluate the treatments. These mice develop pancreatic cancer very rapidly due to genetically engineered mutations of the Kras and P53 genes. The Kras gene is associated with cell proliferation, and the P53 gene is related to tumor suppression [18]. The combination of a ketogenic diet with gemcitabine, a chemotherapeutic drug used to treat various types of cancers, resulted in a significant increase in overall median survival (119 days) of pancreatic cancer patients compared to the gemcitabine alone group (88 days). However, it is important to note that mouse models have limitations, and the results may not directly translate to humans due to species-specific differences in treatment response and underlying mechanisms. Additionally, the relatively small sample size (16-23 mice/group) calls for larger sample sizes to enhance statistical power and result reliability [19]. While both Zahra et al. and Cortez et al. demonstrate the potential of combined chemotherapy and ketogenic diet treatment for pancreatic cancer, they did not account for potential variations due to the diverse tumor subpopulations found in cancer cells. This high diversity might alter the effectiveness of patients' tumors responses to these treatments. Considering tumor heterogeneity could provide a more comprehensive understanding of the observed effects.

In addition to a ketogenic diet, the efficacy of chemotherapy treatments have been enhanced by other approaches. Iyikesici conducted a study involving 25 stage IV pancreatic cancer patients and added hyperbaric oxygen therapy (HBOT) as an additional treatment [20]. During HBOT, a person is placed in a chamber where the atmospheric pressure is increased to a level higher than normal, allowing the lungs to take in more oxygen. HBOT improves chemotherapy effectiveness by increasing oxygen levels in the bloodstream, thereby enhancing oxygen supply to tumor tissues. This helps overcome tumor hypoxia and increases cancer cell sensitivity to chemotherapy. Furthermore, the increased oxygen supply in HBOT can enhance the effectiveness of a ketogenic diet by providing normal cells with oxygen as a substrate for oxidative phosphorylation during ketosis [21]. Improved oxygen delivery supports the oxidative metabolism of ketone bodies, which serve as an energy source for normal cells in place of carbohydrates. In the context of cancer, HBOT helps reduce radiation therapy side effects and supports wound healing [20]. Iyikesici’s study showed that the median overall survival during the mean follow-up period of 25.4 ± 19.3 months was 15.8 months. However, one limitation of Iyikesici's study was the absence of a control group, as they compared their results to historical controls. While Iyikesici’s study did not include patients on chemotherapy treatment alone, Gebbia et al. did, and reported a median overall survival of 6 months (range, 2-8.2 months) for stage IV pancreatic cancer patients [22]. Therefore, the results published by Iyikesici suggest that the addition of HBOT and a ketogenic diet simultaneously could enhance chemotherapy effectiveness.
While preclinical and clinical studies investigating the impact of ketogenic diets combined with chemotherapy have shown more promise compared to chemotherapy alone, further research is necessary to comprehend the long-term efficacy of these treatments. It is important to consider that the mentioned studies focused on pancreatic and lung cancer patients, and the findings may not be generalizable to other cancer types. To explore the effect of combined treatment in different cancer types, a randomized controlled clinical trial has been approved in China in 2019. This trial aims to assess the effects of ketogenic diets combined with chemotherapy on overall survival in 519 women with breast cancer. However, as the study is still ongoing, the results have not yet been published [23].
Metabolic analysis
In addition to assessing overall survival, it is crucial to understand the metabolic changes that occur in cancer patients during combined chemotherapy and ketogenic diet treatment. Metabolic analyses not only examine tumor cells but also shed light on the metabolic interactions within the tumor microenvironment. Tumor cells interact with surrounding stromal cells, immune cells, and blood vessels, creating a complex metabolic ecosystem [24]. Analyzing the metabolic interactions between tumor cells and their microenvironment can deepen our understanding of tumor biology and the potential of a ketogenic diet to overcome solid tumors' resistance to chemotherapy.
Two studies conducted by Zahra et al. and Cortez et al. provide insights into metabolic markers affected by the ketogenic diet [18,19]. Zahra et al. observed significant reductions in blood glucose levels and improvements in insulin sensitivity after implementing the diet. They also noted changes in other metabolic biomarkers, such as decreased levels of branched-chain amino acids and increased levels of ketone bodies. Cortez et al. compared the effects of a ketogenic diet with gemcitabine (KG) to gemcitabine alone (CG) in terms of metabolic markers and inflammation [19]. The KG group showed significantly increased blood ketone levels and improved glucose metabolism compared to the CG group. In males, KG exhibited anti-inflammatory effects by reducing the levels of interferon-gamma (IFN-γ), a pro-inflammatory cytokine. However, KG also had higher levels of KC/GRO chemokines, associated with pancreatic cancer progression [25]. These differences in cytokine and chemokine profiles highlight variations in the inflammatory response.
Safety of a ketogenic diet coupled with chemotherapy
Chemotherapy treatments can be toxic to cancer patients. One side effect is neurotoxicity and peripheral neuropathy, a condition that affects the peripheral nerves located outside of the brain and spinal cord. Beijers et al. highlighted the neurotoxic effects of chemotherapy, including peripheral neuropathy, cognitive impairment, and mood disturbances [26]. Argyriou et al. found that peripheral neuropathy is a common complication of chemotherapy, causing sensory and motor symptoms [27]. Furthermore, Zahra et al. reported that 5 out of the 7 patients with lung cancer enrolled in their study did not complete the study due to a wide range of symptoms associated with the treatment. These symptoms mainly included difficulty complying with the demands of a ketogenic diet, vomiting, fatigue, and hyperuricemia, which is characterized by elevated uric acid levels in the body. Elevated uric acid levels can lead to the formation of sharp crystals that accumulate and increase the risk of kidney stone development [18]. Ma et. al also reported that head and neck cancer patients had difficulty tolerating chemotherapy, with several side effects such as nausea, vomiting, diarrhea, constipation, neutropenic fever, fatigue, hyperuricemia, and pancreatitis [28]. Despite the benefits seen amongst patients following the ketogenic diet coupled with chemotherapy, the potential side effects associated with it cannot be neglected. Further research is needed to fully evaluate the effectiveness and interactions of the ketogenic diet with specific chemotherapy regimens.
Ketogenic diet coupled with immunotherapy vs immunotherapy alone
Overall survival
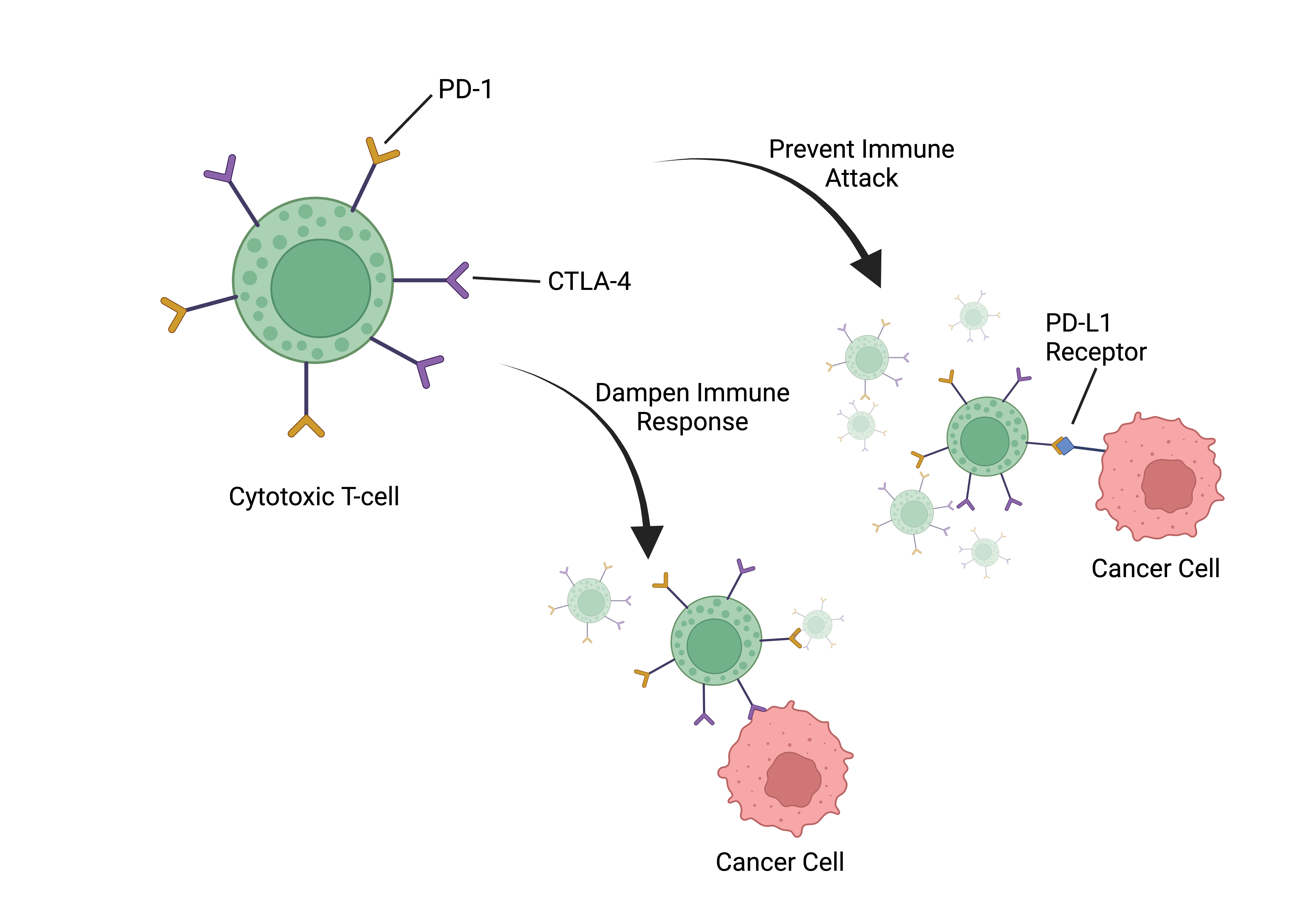
Currently, only one preclinical study by Ferrere et al. has evaluated the efficacy of combining a ketogenic diet (KD) with immune checkpoint blockade (ICB) therapy in cancer treatment [29]. ICBs target specific regulatory pathways, known as checkpoints, to enhance the immune response and maintain immune system balance. Cancer cells exploit immune checkpoints to evade immune attacks, but ICBs release the brakes on the immune system, enabling it to recognize and attack cancer cells effectively. PD-1 (programmed cell death protein 1) and CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) are two well-known targeted immune checkpoint proteins expressed on the surface of immune cells, particularly T cells. These proteins play critical roles in regulating the immune response. PD-1 helps prevent T cells from launching an excessive immune attack by interacting with its ligands, such as PD-L1, which are often expressed on tumor cells. CTLA-4, on the other hand, primarily operates during the early activation of T cells, serving to dampen immune responses and maintain immune system balance. In cancer, these checkpoints are often exploited by tumors to evade immune surveillance, making them attractive targets for immunotherapy [16]. Blocking PD-1 and CTLA-4 receptors with antibodies or drugs prevents immune suppression and enhances the anti-tumor response of cytotoxic T cells - white blood cells involved in the specific detection and eradication of cancer cells.
According to Ferrere et al., combining a ketogenic diet with ICB therapy showed improved tumor control in established renal cancers, particularly when BALB/c mouse hosts - often used in immunology research - were treated with intermittent KD or the ketone body 3HB [21]. The same study also showed long-term survival in the majority of C57BL/6J mice treated with anti-PD-1 and a KD. Ferrere et. al also demonstrated that in an aggressive orthotopic TC-1 lung cancer model, resistant to standalone anti-PD-1 or anti-CTLA-4 therapies, intermittent administration of 3HB and anti-PD-1 antibodies resulted in over 70% eradication of established tumors. In comparison, the use of anti-PD-1 antibodies alone resulted in eradication in only 35% of cases [29].
As well, the combination therapy led to long-term survival in the majority of C57BL/6J hosts. Furthermore, mice that were cured of TC-1 lung cancers using the combination of 3HB and anti-PD-1 therapy showed resistance when rechallenged with TC-1 cancers, indicating the development of a specific long-term protective immune response. The data suggests that the synergy between ketogenic regimens and immune checkpoint blockade (ICB) therapy amplifies the anticancer effects across diverse orthotopic tumor mouse models, such as renal cancer and non-small cell lung cancer, where tumors are placed in their native tissue locations within the mice, replicating the natural growth environment within the body.
Conclusion
This review focuses on the potential of ketogenic diets (KDs) as an additional therapy for cancer treatment with an emphasis on solid tumors. According to the research, combining a ketogenic diet with immunotherapy or chemotherapy has the potential to increase overall survival rates and treatment effectiveness in comparison to these therapies alone. With improved treatment responses, tumor reduction, and disease control, a number of studies—including preclinical and clinical trials—support the addition of a ketogenic diet to chemotherapy. Furthermore, patients who received combined treatments had a higher overall survival percentage than those who received chemotherapy alone. Metabolic tests showed significant dropped blood glucose levels, increased insulin sensitivity, and changed metabolic indicators. However, safety studies have shown many potential side effects not limited to neurotoxicity, nausea, and hyperuricemia.
The findings of this literature evaluation confirm the need for additional studies to fully assess the long-term efficacy of treatments that include ketogenic diets. To determine the generalizability of the effects of ketogenic diets, studies should also examine their effects in a variety of cancer types and patient demographics. Clinical trials with randomization and control groups are necessary to verify the results and produce stronger proof of efficacy. To further understand the effects of ketogenic diets, future research should also take tumor heterogeneity and metabolic interactions within the tumor microenvironment into account. Future investigation and clinical application of ketogenic diets as supplementary cancer therapies promises to be a fascinating field. Further research into how ketogenic diets affect metabolism and immunity is necessary, given the pressing need for novel therapeutic approaches to improve patient outcomes and survival rates. Finally, understanding the processes underlying the beneficial interactions between chemotherapy and immunotherapy and ketogenic diets will aid in the creation of individualized and successful cancer treatment modalities.

About the Author: Tarek Bacha
Tarek Bacha is a UC Davis alumnus who graduated in the Spring of 2023 with a B.S. in Neurobiology, Physiology, and Behavior. As a scientist conducting cancer research, Tarek believes that studying human physiology would provide him with a better understanding of the underlying complications related to cancer. During his undergraduate journey, he was actively involved in nutritional cancer research with a specific emphasis on ketogenic diets. His determination to broaden his knowledge on this topic led him to publish a literature review in Aggie Transcript. This review offers readers the opportunity to comprehend ketogenic diets from a biochemical perspective and how they can potentially be integrated to enhance current cancer treatments. Tarek's ultimate goal is to pursue an MD/PhD degree with a focus on integrative medicine. He aspires to continue his research in cancer, while also remaining open to exploring other research avenues. Given his ambitious nature, he humorously acknowledges that winning a Nobel Prize wouldn't be unwelcome. Lastly, Tarek wishes to express his gratitude to the Islamic Center of Davis (ICD), where he dedicated 2-3 years as a volunteer. The Muslim community at ICD provided him with a lasting sense of belonging and nurtured his growth as a leader. It also equipped him with the foundational knowledge necessary for his personal and academic success.
Author's Note
This literature review was written as part of my UWP 104FY course. The intended audience is scientists, including undergraduate research students. The focus of this review is on current cancer treatments and the impact of nutrition on their efficacy. The aim is to demonstrate the potential of ketogenic diets as adjunctive therapies to chemotherapy and immunotherapy, acknowledging the limited availability of clinical data.
References
- Neal, Elizabeth G et al. 2008. “The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial.” The Lancet. Neurology. vol. 7,6: 500-6.
- Alexander, James L et al. 2017. “Gut microbiota modulation of chemotherapy efficacy and toxicity.” Nature reviews. Gastroenterology & hepatology. vol. 14,6: 356-365.
- Bader, Jackie E et al. 2020. “Targeting Metabolism to Improve the Tumor Microenvironment for Cancer Immunotherapy.” Molecular cell. vol. 78,6: 1019-1033.
- Greathouse, K Leigh et al. 2022. “Diet-microbiome interactions in cancer treatment: Opportunities and challenges for precision nutrition in cancer.” Neoplasia. vol. 29: 100800.
- Yudkoff, Marc et al. 2007. “The ketogenic diet and brain metabolism of amino acids: relationship to the anticonvulsant effect.” Annual review of nutrition. vol. 27: 415-30.
- Smyl, Christopher. 2016. “Ketogenic Diet and Cancer-a Perspective.” Recent results in cancer research. Fortschritte der Krebsforschung. Progres dans les recherches sur le cancer. vol. 207: 233-40.
- Kong, Cheng et al. 2021. “Ketogenic diet alleviates colitis by reduction of colonic group 3 innate lymphoid cells through altering gut microbiome.” Signal transduction and targeted therapy. vol. 6,1 154.
- Pinto, Alessandro et al. 2018. “Anti-Oxidant and Anti-Inflammatory Activity of Ketogenic Diet: New Perspectives for Neuroprotection in Alzheimer's Disease.” Antioxidants. vol. 7,5 63.
- Skinner, Robert et al. 2009. “Ketone bodies inhibit the viability of human neuroblastoma cells.” Journal of pediatric surgery. vol. 44,1: 212-6.
- Cortez, Natalia E, and Gerardo G Mackenzie. 2021. “Ketogenic Diets in Pancreatic Cancer and Associated Cachexia: Cellular Mechanisms and Clinical Perspectives.” Nutrients. vol. 13,9 3202.
- Liberti, Maria V, and Jason W Locasale. 2016. “The Warburg Effect: How Does it Benefit Cancer Cells?.” Trends in biochemical sciences. vol. 41,3: 211-218.
- Martinez-Outschoorn, Ubaldo E et al. 2012. “Ketone body utilization drives tumor growth and metastasis.” Cell cycle. vol. 11,21: 3964-71.
- Sriraman, Shravan Kumar et al. 2014. “Barriers to drug delivery in solid tumors.” Tissue barriers. vol. 2 e29528.
- Scott, Ellen N et al. 2021. “Regulatory T Cells: Barriers of Immune Infiltration Into the Tumor Microenvironment.” Frontiers in immunology. vol. 12 702726.
- Eddy, Kevinn, and Suzie Chen. 2020. “Overcoming Immune Evasion in Melanoma.” International journal of molecular sciences. vol. 21,23 8984.
- Zhang, Yuanyuan, and Zemin Zhang. 2020. “The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications.” Cellular & molecular immunology. vol. 17,8: 807-821.
- Weber, Daniela D et al. 2020. “Ketogenic diet in the treatment of cancer - Where do we stand?” Molecular metabolism. vol. 33 (2020): 102-121.
- Zahra, Amir et al. 2017. “Consuming a Ketogenic Diet while Receiving Radiation and Chemotherapy for Locally Advanced Lung Cancer and Pancreatic Cancer: The University of Iowa Experience of Two Phase 1 Clinical Trials.” Radiation research vol. 187,6: 743-754.
- Cortez, Natalia E et al. 2022. “A ketogenic diet in combination with gemcitabine increases survival in pancreatic cancer KPC mice.” Cancer research communications. vol. 2,9: 951-965.
- Iyikesici, Mehmet Salih. 2020. “Long-Term Survival Outcomes of Metabolically Supported Chemotherapy with Gemcitabine-Based or FOLFIRINOX Regimen Combined with Ketogenic Diet, Hyperthermia, and Hyperbaric Oxygen Therapy in Metastatic Pancreatic Cancer.” Complementary medicine research. vol. 27,1: 31-39.
- Poff, Angela M et al. 2013. “The ketogenic diet and hyperbaric oxygen therapy prolong survival in mice with systemic metastatic cancer.” PloS one. vol. 8,6 e65522.
- Gebbia, Vittorio et al. 2010. “Irinotecan plus bolus/infusional 5-Fluorouracil and leucovorin in patients with pretreated advanced pancreatic carcinoma: a multicenter experience of the Gruppo Oncologico Italia Meridionale.” American journal of clinical oncology. vol. 33,5: 461-4.
- Wang, Yan et al. 2020. “Does a ketogenic diet as an adjuvant therapy for drug treatment enhance chemotherapy sensitivity and reduce target lesions in patients with locally recurrent or metastatic Her-2-negative breast cancer? Study protocol for a randomized controlled trial.” Trials. vol. 21,1 487.
- Mao, Xiaoqi et al. 2021. “Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives.” Molecular cancer. vol. 20,1 131.
- Halbrook, Christopher J et al. 2019. “Macrophage-Released Pyrimidines Inhibit Gemcitabine Therapy in Pancreatic Cancer.” Cell metabolism vol. 29,6: 1390-1399.e6.
- Beijers, A J M et al. 2012. “Chemotherapy-induced neurotoxicity: the value of neuroprotective strategies.” The Netherlands journal of medicine. vol. 70,1: 18-25.
- Argyriou, A A et al. 2010. “Toxic peripheral neuropathy associated with commonly used chemotherapeutic agents.” Journal of B.U.ON. : official journal of the Balkan Union of Oncology. vol. 15,3: 435-46.
- Ma, Daniel C et al. 2021. “Ketogenic Diet with Concurrent Chemoradiation in Head and Neck Squamous Cell Carcinoma: Preclinical and Phase 1 Trial Results.” Radiation research. vol. 196,2: 213-224.
- Ferrere, Gladys et al. 2021. “Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade.” JCI insight. vol. 6,2 e145207.
