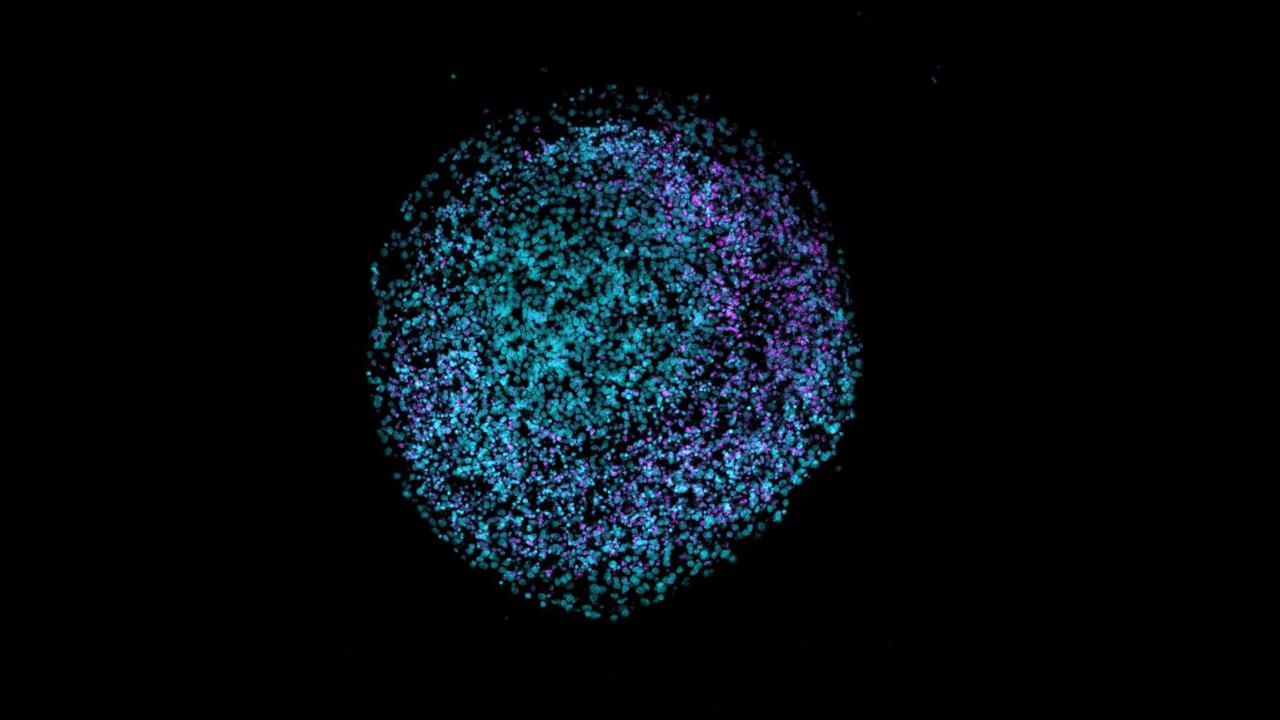
Modeling Alzheimer’s Disease Using Human Brain Organoids
Introduction
Alzheimer’s Disease (AD) is a progressive neurodegenerative disease that starts with mild memory loss and progresses to the loss of the ability to carry out daily activities, ultimately leading to death. AD continues to affect more and more people worldwide. At least 55 million people were living with AD in 2020, and that number is projected to reach 78 million in 2030 [1]. Despite the speed at which the number of AD patients is growing, there is still a lack of effective treatment and understanding of the origin of this disease.
In order to increase our understanding of AD’s pathophysiology, various models have been used in the past, such as transgenic animals and 2-dimensional human induced pluripotent stem cell (iPSC) derived models. However, these models are limited and fail to exhibit the various nuances of the human brain. Recent advances in stem cell research have led to the development of in vitro self-assembling 3-dimensional models of the human brain, called human brain organoids or cerebral organoids [2]. Human brain organoids can model pathological processes of AD, including amyloid plaques, neurofibrillary tangles, brain atrophy, and inflammation [3]. Furthermore, these organoids may be used to assess the effectiveness of various drugs for AD [4]. This literature review will synthesize the research done on the use of 3D human brain organoids to model AD pathology and its use in developing and assessing treatments for AD.
Alzheimer’s Disease Background
The ultimate goal of modeling AD in brain organoids is to use them as a tool to develop new therapies for patients with AD. Therefore, it is important to know the pathology of AD to ensure that these brain organoids will fully replicate those key characteristics. The two main characteristics of AD are the aggregation of amyloid-β (Aβ) peptides and hyperphosphorylated tau proteins in the brain. Aꞵ peptides are segments of the amyloid precursor protein which aggregate into plaques in the brain. These protein aggregates can trigger neurofibrillary tangles (NFTs), neuron apoptosis (cell death), and neuroinflammation (inflammation of the brain), ultimately disrupting normal neuron activity [5].
Aꞵ plaques are formed through a series of proteolytic cleavages of the amyloid precursor protein (APP) by the ꞵ-secretase and the 𝛾-secretase enzymes [6, 7]. Mutations in the APP, presenilin 1 (PSEN1), and presenilin 2 (PSEN2) genes affect Aβ formation because PSEN1 and PSEN2 encode essential catalytic subunits of the 𝛾-secretase, while APP mutation encourages its cleavage into Aꞵ plaques [7, 8]. Amyloid plaques are problematic because they accumulate extracellularly in the brain and disrupt synaptic connections between cells [9], leading to impaired neural communication in the brain.
Normally, tau proteins stabilize neuron microtubules through constant phosphorylation and dephosphorylation. However, with AD, hyperphosphorylated tau proteins accumulate intracellularly, forming neurofibrillary tangles (NFTs). These NFTs disrupt axonal transport (the use of microtubules to deliver materials through the axon), compromise the integrity of the cytoskeleton, lead to synapse dysfunction, and can cause cell death [7, 10].
Additionally, the apolipoprotein (ApoE) gene is associated with both familial AD (FAD) and sporadic AD (SAD). More specifically, the ε4 allele of the ApoE4 isoform is the strongest genetic risk factor for AD [5, 11]. This genetic link underscores the complex interplay of genetic factors in the development of AD.
Genetic factors, such as mutations in APP, PSEN1, PSEN2, and the ApoE ε4 allele, contribute significantly to AD pathogenesis and are crucial considerations for creating organoids that accurately replicate features of AD.
Brain Organoid Technology

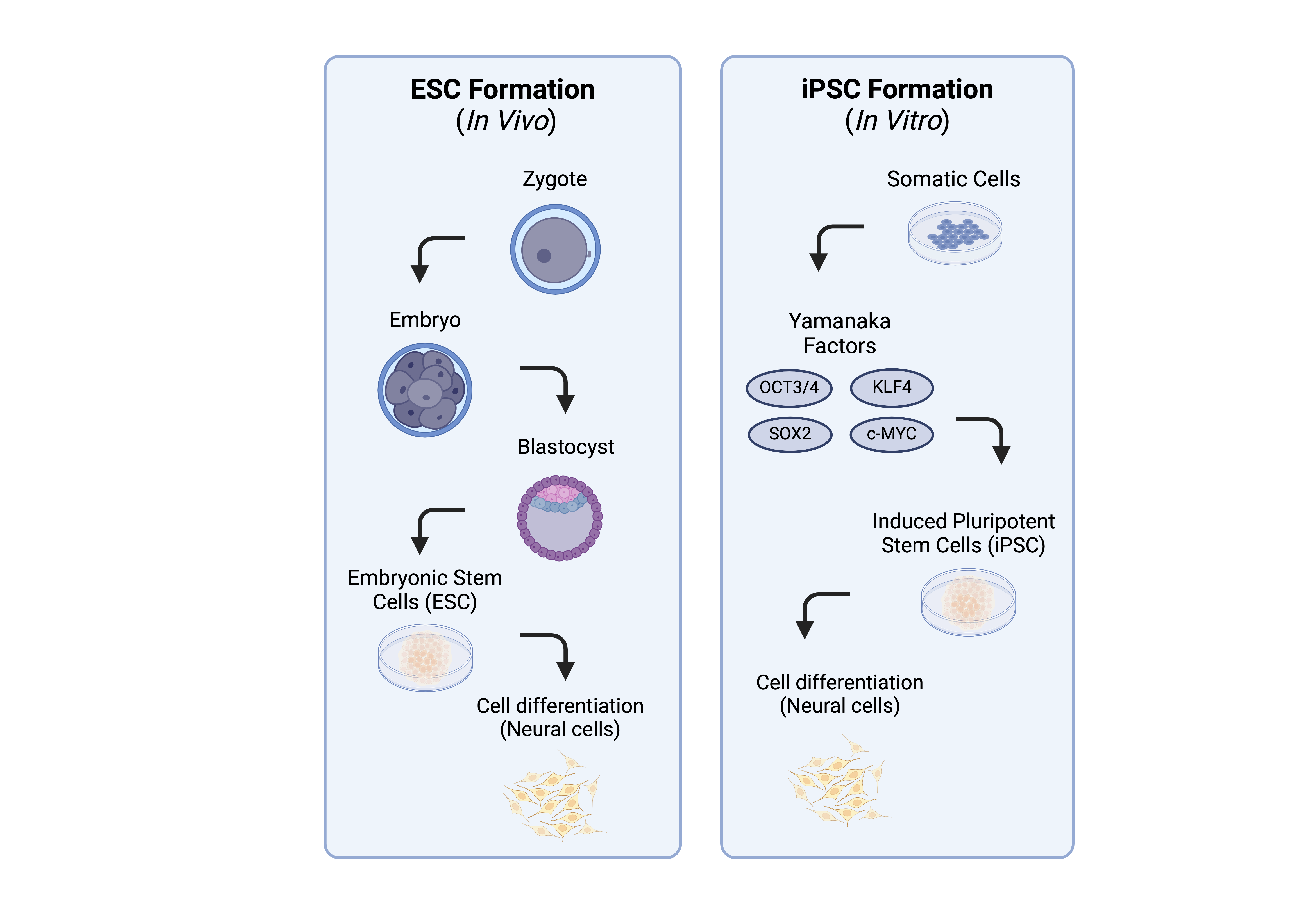
The development of human brain organoids, also referred to as cerebral organoids, offers a powerful tool for studying the intricacies of the human brain in a controlled laboratory setting. Organoids are made from induced pluripotent stem cells (iPSCs), which exhibit characteristics akin to embryonic stem cells such as dividing indefinitely and self-organizing into various cell types [5, 9].
In 2013, Lancaster et al. marked a milestone by successfully culturing a three-dimensional brain tissue that displays discrete regions that resemble a human brain [12]. Researchers successfully modeled human microcephaly (a condition in which the head is small due to underdevelopment of the brain) with human brain organoids, demonstrating a potential to replicate the genetic characteristics of stem cell donors. Creating donor-specific organoids can aid in the process of understanding an individual’s disease, therefore allowing doctors to create an effective specialized plan of treatment for patients. The process involves initiating growth in a three-dimensional neuroectodermal tissue structure (embryonic tissue that gives rise to nervous structure), followed by the addition of Matrigel droplets, a growth factor, to the brain tissue in a spinning bioreactor to enhance nutrient absorption. Distinct brain regions can be identified through specific genetic markers for each area, such as FOXG1 and SIX3 for forebrain regions and KROX20 and ISL1 for hindbrain regions [12]. Notably, human-specific cortical features, including the outer subventricular zone (OSVZ) and the inner fiber layer (IFL) that divides the SVZ, are observed in cerebral organoids, setting them apart from mouse organoids. The differences between mice models and human iPSC-derived models are particularly apparent in microglia, astrocytes, oligodendrocytes, and brain vasculature [12, 13].
AD pathophysiology in brain organoids
Aβ Plaque
Various organoid models have been engineered to investigate the formation of Aβ plaques in AD. This has provided valuable insight into the role of various genetic factors in Aβ plaque formation and its relevance to AD pathogenesis.
Brain organoids using iPSCs from FAD patients with APP duplication, PSEN1 mutations, or PSEN2 mutations showed significantly higher levels of Aβ aggregates and an increased ratio of Aβ42/Aβ40 compared to the control. It was also found that Aβ aggregates increase in size as time goes on, demonstrating the progressive nature of Aβ plaque formation in AD [14, 8]. In another experiment that investigated FAD risk genes, four groups of cerebral organoids were researched: control carrying APOE ε3/ε3, control carrying APOE ε4/ε4, SAD carrying ε3/ε3, and SAD carrying ε4/ε4. The Aβ42/Aβ40 ratio is also increased in all AD organoids independent of APOE4, leading researchers to believe that the increase in Aβ plaques is due to a problem with the Aβ clearance mechanism rather than an increase in APP cleavage [11].
Some researchers looked at Down syndrome (DS)-derived organoids to observe Aβ metabolism because DS is characterized by an extra copy of chromosome 21, which harbors the APP gene. These organoids revealed heightened levels of proteins involved in Aβ prevention and degradation, offering valuable insights into how chromosome 21 triplication impacts Aβ formation [6]. Additionally, cerebral organoids from DS patients displayed Aβ plaques similar in size and morphology to those observed in AD patients’ brains, highlighting the relevance of DS-derived models in understanding Aβ pathology [9].
Lastly, organoids derived from iPSCs with a loss-of-function mutation of the pitrilysin metallopeptidase 1 (PITRM1) gene showed increased levels of Aꞵ plaque accumulation and tau hyperphosphorylation through western blotting, immunoassays, and immunofluorescence staining [15]. PITRM1 is a mitochondrial protease found to be associated with AD in the presence of mutations. This study by Pérez et al. illuminates the significance of mitochondrial dysfunction in Aβ plaque clearance mechanisms and tau-related pathogenesis in AD pathology.
Hyperphosphorylated Tau and Neurofibrillary Tangles
Aggregation of hyperphosphorylated tau proteins into NFTs was also replicated in human brain organoids derived from iPSCs with various genetic modifications. Unlike the Aβ plaque formation found in FAD organoids with an APP or PSEN1 mutation, phosphorylated tau (p-tau) levels do not increase as time progresses, despite overall higher p-tau levels compared to control organoids. This observation regarding the timing of Aβ and p-tau formation indicates that these pathological features are related and may augment each other's characteristics [14]. Moreover, organoids carrying the APOE4 allele display elevated tau levels regardless of AD status. This finding emphasizes the role of APOE4 as a genetic risk factor for both FAD and SAD [11].
Interestingly, when aggregation of p-tau in the form of NFTs was compared among various organoids from iPSCs of patients with AD, DS, Creutzfeldt-Jakob disease, and healthy individuals, DS organoids showed the most NFTs. FAD organoids followed as a close second [9]. This shows that DS patients may have some genes that increase the risk of AD-like characteristics in their brains, increasing their likelihood of showing AD-like symptoms.
Neuron Apoptosis and Brain Atrophy

Many brain organoids can also model brain atrophy, which occurs due to an increase in neural apoptosis. Cleaved caspase-3 (CASP3) activity is a widely used marker to detect apoptosis in various cellular and organoid models. CASP3 is an enzyme involved in the execution phase of apoptosis; it plays a pivotal role in the breakdown of cellular components, ultimately leading to cell death [8]. High levels of CASP-3 activity have been detected in organoids with the APOE4 allele [11], DS [9], the PSEN2 mutation [8], and PITRM1-knockout [15]. This reveals that these genes may be involved in the atrophy process observed in AD patients.
Inflammation
In addition to Aβ-plaque formation and neurofibrillary tangles (NFTs), neuroinflammation is an essential pathology of AD and other neurodegenerative disorders. Microglia promote AD-related neuroinflammatory responses like chemokine release and reduce the survival rate of neurons and astrocytes in AD environments. This phenomenon was demonstrated by Park et al. (2018) when they engineered an organoid model to demonstrate neuron, astrocyte, and microglia interaction [16]. When microglial cells were placed in a chamber adjacent to a chamber with Neu+AC, the microglia migrated towards the Neu+AC chamber in 48 hours, resulting in inflammatory responses. These results reveal the significance of the interactions among various neurological cell types in the development and progression of AD [16].
Limitations
Organoid Technology
Human brain organoids have become the focus of numerous neurodevelopmental research due to their ability to model the intricate process of brain formation in embryos. Therefore, it is essential to acknowledge the limitations associated with using brain organoids for modeling neurodegenerative diseases, despite their growing popularity in this context [17]. Additionally, multiple technological limitations of brain organoid technology pose significant challenges to its utility in modeling neurological diseases and disorders. This requires further advancements in the field.
One of the biggest challenges is the lack of certain specialized cells critical for modeling neurological conditions. Microglial cells, for instance, are important for modeling AD because they can be stimulated by Aβ proteins to cause neuron death, damage synapses, and change neurotransmitter activity [5].
The absence of a vascular system is another problem, as it is necessary to supply nutrients and oxygen to deeper layers of organoid tissue. Therefore, one of the challenges encountered in the study of brain organoids is the characterization of necrosis in their central regions [14]. Vasculature is also related to specific AD pathogenesis such as regulation of Aβ and hyperphosphorylated tau formation, thus it is important to understand the full scope of AD pathology [5].
The blood brain barrier (BBB), an extension of the vasculature, is particularly important for the development and testing of anti-AD drugs. It acts as a filter to prevent molecules from entering the brain [5, 18]. Therefore, the inclusion of the BBB in brain organoids will allow for improved drug discovery and testing, providing insights into the brain's response to potential treatments. Recent advances have demonstrated progress in this area, with Nzou et al. (2018) successfully incorporating multiple cell types (microvascular endothelial cells, pericytes, astrocytes, microglia, oligodendrocytes, and neurons) into their organoid model to mimic BBB effects [18].
Another limitation is the disconnection of the organoid from other organs, especially the gut. Mounting evidence suggests that gut microbes can influence brain chemistry, with disruptions potentially leading to neuroinflammation and amyloidosis [5].
Ethics
As research on brain organoids advances, concerns about ethical issues are arising among scientists, donors, and the public. Most researchers obtain biomaterials for brain organoids from donors following guidelines published by the International Society for Stem Cell Research, called “Guidelines for Stem Cell Science and Clinical Translation”. Another concern is the effectiveness of translating the results from studies on organoids to a living patient. The biggest debate surrounding the advancement of brain organoids is the possible emergence of consciousness and the formation of self-awareness in these organoids [19]. However, Hyun et al. point out that an actual human brain is much more complex than an organoid, so it is highly unlikely that consciousness will form.
Conclusion
In addition to advancing our understanding of AD, organoids have emerged as a promising alternative to animal models for testing and screening drugs. They are more advantageous because they are more "human-like", and thousands of organoids can be maintained in a smaller space [9]. New anti-AD drugs can be administered to human brain organoids displaying AD pathology to assess their effectiveness. For example, when β and 𝛾-secretase inhibitors were introduced to FAD organoids, Aβ aggregates and p-tau levels decreased [14]. In another study, APOE4 was converted to APOE3. This resulted in many of the AD pathologies being reduced such as CASP3 activity, accumulation of Aβ40 and Aβ42, and p-tau levels [11].
In the future, cerebral organoids may become the treatment for AD through direct transplantation into the brain to reconstruct damaged neural networks in patients with neurological diseases [5]. Experiments transplanting organoids derived from human iPSCs into mice and monkeys have shown success. The grafted tissue exhibits signs of differentiation, extension of axons, bidirectional synaptic connections, and functions as mature neurons in the host’s brain [20, 21].
The prospect of brain organoids serving as a platform for drug screening and transplantation therapies underscores the critical role they can play in advancing our efforts to combat AD and other neurodegenerative diseases. Continued research is crucial in discovering effective treatments and potential cures for AD in the future.

About the Author: Yoonah Kang
Yoonah is a third year student studying Neurobiology, Physiology, and Behavior. She always enjoyed biology in middle school and high school. Her interest in neurobiology began in high school when she took AP psychology. This paper was originally written for the UWP 104E class, writing in research. She has already written a paper about Alzheimer’s disease, which led to the interest in ways to model AD for research. She came upon brain organoid technology and was fascinated by how it models AD using human cells. After graduation, Yoonah plans to apply to physician assistant programs.
References
1. BrightFocus Foundation. (2022, October 5). Alzheimer’s disease: Facts & figures. BrightFocus. https://www.brightfocus.org/alzheimers/article/alzheimers-disease-facts-figures
2. Lullo, E. D., and Kriegstein, A. R. (2017). The use of brain organoids to investigate neural development and disease. Nature, 18. https://doi.org/10.1038/nrn/2017/107
3. Yanakiev, M., Soper, O., Berg, D. A., & Kang, E. (2023). Modelling Alzheimer’s disease using human brain organoids: Current progress and challenges. Expert Reviews in Molecular Medicine, 25(3), 1-12. https://doi.org/10.1017/erm/2022/40
4. Papaspyropoulos, A., Tsolaki, M., Foroglou, N., & Pantazaki, A. A. (2020). Modeling and targeting Alzheimer’s disease with organoids. Frontiers in Pharmacology, 11. https://doi.org/10.3389/fphar.2020.00396
5. Bi, F. C., Yang, X. H., Cheng, X. Y., Deng, W. B., Guo, X. L., Yang, H., ... & Yao, Y. (2021). Optimization of cerebral organoids: a more qualified model for Alzheimer's disease research. Translational Neurodegeneration, 10(27). https://doi.org/10.1186/s40035-021-00252-3
6. Alić, I., Goh, P. A., Murray, A., Portelius, E., Gkanatsiou, E., Gough, G., ... & Nižetić, D. (2021). Patient-specific Alzheimer-like pathology in trisomy 21 cerebral organoids reveals BACE2 as a gene dose-sensitive AD suppressor in human brain. Molecular psychiatry, 26(10), 5766-5788. https://doi.org/10.1038/s41380-020-0806-5
7. Shepherd, C., McCann, H., & Halliday, G. M. (2009). Variations in the neuropathology of familial Alzheimer’s disease. Acta neuropathologica, 118, 37-52. https://doi.org/10.1007/s00401-009-0521-4
8. Yin, J., & VanDongen, A. M. (2020). Enhanced neuronal activity and asynchronous calcium transients revealed in a 3D organoid model of Alzheimer’s disease. ACS Biomaterials Science & Engineering, 7(1), 254-264. https://doi.org/10.1021/acsbiomaterials.0c01583
9. Gonzalez, C., Armijo, E., Bravo-Alegria, J., Becerra-Calixto, A., Mays, C. E., & Soto, C. (2018). Modeling amyloid beta and tau pathology in human cerebral organoids. Molecular psychiatry, 23(12), 2363-2374. https://doi.org/10.1038/s41380-018-0229-8
10. Choi, H., Kim, H. J., Yang, J., Chae, S., Lee, W., Chung, S., ... & Mook‐Jung, I. (2020). Acetylation changes tau interactome to degrade tau in Alzheimer’s disease animal and organoid models. Aging Cell, 19(1), e13081. https://doi.org/10.1111/acel.13081
11. Zhao, J., Fu, Y., Yamazaki, Y., Ren, Y., Davis, M. D., Liu, C. C., ... & Bu, G. (2020). APOE4 exacerbates synapse loss and neurodegeneration in Alzheimer’s disease patient iPSC-derived cerebral organoids. Nature communications, 11(1), 5540. https://doi.org/10.1038/s41467-020-19264-0
12. Lancaster, M. A., Renner, M., Martin, C. A., Wenzel, D., Bicknell, L. S., Hurles, M. E., … & Knoblich, J. A. (2013). Cerebral organoids model human brain development and microcephaly. Nature, 501(7467), 373-379. https://doi.org/10.1038/nature12517
13. Cerneckis, J., Bu, G., & Shi, Y. (2023). Pushing the boundaries of brain organoids to study Alzheimer’s disease. Trends in Molecular Medicine. https://doi.org/10.1016/j.molmed.2023.05.007
14. Raja, W. K., Mungenast, A. E., Lin, Y. T., Ko, T., Abdurrob, F., Seo, J., & Tsai, L. H. (2016). Self-organizing 3D human neural tissue derived from induced pluripotent stem cells recapitulate Alzheimer’s disease phenotypes. PloS one, 11(9), e0161969. https://doi.org/10.1371/journal.pone.0161969
15. Pérez, M. J., Ivanyuk, D., Panagiotakopoulou, V., Di Napoli, G., Kalb, S., Brunetti, D., ... & Deleidi, M. (2021). Loss of function of the mitochondrial peptidase PITRM1 induces proteotoxic stress and Alzheimer’s disease-like pathology in human cerebral organoids. Molecular psychiatry, 26(10), 5733-5750. https://doi.org/10.1038/s41380-020-0807-4
16. Park, J., Wetzel, I., Marriott, I., Dréau, D., D’Avanzo, C., Kim, D. Y., ... & Cho, H. (2018). A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nature neuroscience, 21(7), 941-951. https://doi.org/10.1038/s41593-018-0175-4
17. Mulder, L. A., Depla, J. A., Sridhar, A., Wolthers, K., Pajkrt, D., & Vieira de Sá, R. (2023). A beginner’s guide on the use of brain organoids for neuroscientists: a systematic review. Stem Cell Research & Therapy, 14(1), 87. https://doi.org/10.1186/s13287-023-03302-x
18. Nzou, G., Wicks, R. T., Wicks, E. E., Seale, S. A., Sane, C. H., Chen, A., ... & Atala, A. J. (2018). Human cortex spheroid with a functional blood brain barrier for high-throughput neurotoxicity screening and disease modeling. Scientific reports, 8(1), 1-10. https://doi.org/10.1038/s41598-018-25603-5
19. Hyun, I., Scharf-Deering, J. C., & Lunshof, J. E. (2020). Ethical issues related to brain organoid research. Brain research, 1732, 146653. https://doi.org/10.1016/j.brainres.2020.146653
20. Dong, X., Xu, S. B., Chen, X., Tao, M., Tang, X. Y., Fang, K. H., ... & Liu, Y. (2021). Human cerebral organoids establish subcortical projections in the mouse brain after transplantation. Molecular psychiatry, 26(7), 2964-2976.
21. Kitahara, T., Sakaguchi, H., Morizane, A., Kikuchi, T., Miyamoto, S., & Takahashi, J. (2020). Axonal extensions along corticospinal tracts from transplanted human cerebral organoids. Stem Cell Reports, 15(2), 467-481.
