
Dienogest’s Impact on Physiological Parameters: A Comparative Review of Endometriosis Drug Treatments
Introduction
Approximately 10% of reproductive-age biological women struggle with endometriosis [2,3,4,5], a debilitating uterine condition characterized by the atypical growth of endometrial tissue outside of the internal uterine cavity [3] due to imbalances in estrogen production [2,4,5]. Primarily produced in the ovaries, the estrogen hormone is responsible for maintaining many aspects of female reproductive physiology including the growth of endometrial tissue, which lines the internal uterine cavity and thickens during the menstrual cycle [11]. For individuals with endometriosis, the abnormal growth of endometrial tissue beyond the uterine cavity causes various symptoms from dysmenorrhea (extreme menstruation pain) to chronic pelvic pain [3] to decreased fertility [6]. Unfortunately, current pharmaceutical treatment options for endometriosis which regulate estrogen production are associated with a variety of toxic side effects. For example, one popular endometriosis drug is the combined oral contraceptive pill (COCP) which is correlated with an increased risk of thrombotic events, or blood clots [4]. Another popular option is the gonadotropin-releasing hormone (GnRH) injection treatment, which restricts estrogen production but is widely associated with adverse hypoestrogenic reactions such as decreased bone mineral density and decreased libido [3,6]. Beyond their considerable toxic side effects, these widely used pharmaceutical treatment options also continue to raise uncertainty about their efficacy in reducing endometrial symptoms. However, recent emerging research suggests that the drug dienogest may be a noteworthy competitor in regard to treatment efficacy compared to COCP and GnRH. Dienogest is an oral progestin hormone treatment, a synthetic form of the naturally-produced progesterone hormone [12] which—like estrogen—maintains uterine health during the menstrual cycle and pregnancy. Dienogest regulates both progesterone and estrogen production to inhibit pain and endometrial growth [1,2,3,4,5,6,7] and may bear fewer side effects compared to the other two treatments due to its heightened selectivity for progesterone receptors [3]. In addition, dienogest’s lower financial cost compared to GnRH may make it significantly more accessible and appealing to many endometriosis patients.
This review evaluates the efficacy of dienogest as a treatment for endometriosis in comparison to the two most common treatment options: the combined oral contraceptive pill (COCP) and the gonadotropin-releasing hormone (GnRH). The comparison will be drawn through the following parameters: reduction of endometrial tissue, fertility as demonstrated through in-vitro fertilization and embryo transfer (IVF-ET) procedures, and impact on quality of life (QoL) as evaluated through clinical measures of physiological function and personal wellbeing.
Reduction of Endometrial Tissue

All three treatments covered in this review work to resolve estrogen imbalances in the body in order to reduce the growth of endometrioma cysts. Both COCP and GnRH compounds fully suppress estrogen production in the ovaries to prevent new endometriomas from developing. In addition to suppressing estrogen, dienogest targets and suppresses cell progesterone receptors to allow for pain suppression, prevention of cyst recurrence, and the atrophy of existing cyst tissue [3]. In one study, researchers in Osaka, Japan analyzed cyst recurrence rates in patients treated with dienogest following surgical excision of existing cysts [1]. They found that the recurrence rate in the control group grew from 16.5% 12 months after surgery to 24.0% after 24 months [1]. On the other hand, no recurrences in endometriomas were reported in any of the dienogest-treated patients who returned for consistent follow-ups [1]. Although the diminished pool of treated patients who returned for follow-up (just 37.5% of the original group) may be considered a major limitation, the statistically significant difference in recurrence rates (p=0.0025) [1] still clearly supports the implication that dienogest has a significant impact on preventing new cyst growth.
Another study takes a closer look at microscopic-level details of cystic tissue to compare treatment efficacy [2]. Researchers studied the following three biomarkers: the Ki67 protein as an indicator of actively growing cells; the aromatase enzyme signifying estrogen production; and the von Willebrand factor protein, a gauge of blood vessel activity in cystic cells. A decrease in blood vessel activity would directly indicate a treatment’s efficacy in reducing endometrial cells. After sampling endometrial tissue both from patients treated with dienogest and control group (no drug treatment) patients, the researchers found significantly lower (p<0.05) levels of both Ki67 protein and aromatase expression in dienogest-treated endometrial tissue compared to control tissue samples [2]. The lower levels of these two biomarkers suggest that dienogest has a significant impact on re-regulating estrogen production and active endometrial cell growth. However, there was a non-significant difference in von Willebrand factor expression between the two groups [2], suggesting that dienogest did not have the same efficacy in diminishing endometrial blood vessels as it did in preventing cell proliferation and decreasing excess estrogen production. Given that dienogest may not have an impact on blood vessel activity, it would be beneficial for future research to investigate whether or not COCP and GnRH have an effect. In doing so, results can be compared to see if any single treatment is superiorly effective in all three regards (endometrial blood vessel decay, prevention of cystic cell growth, and decreased estrogen production).
A 2021 study directly compared dienogest to COCP by evaluating endometrioma size in treated patients at the Policlinico Umberto I University Hospital in Rome, Italy over the course of 6 months [4]. Researchers observed a significant decrease in dienogest-treated cyst size from 38.3 mm to 25.9 mm [4], while COCP-treated cysts demonstrated no significant size reduction at the trial’s conclusion [4]. These results strongly point to dienogest’s superior efficacy over COCP for the particular concern of diminishing existing cysts. Additionally, the researchers found a significant minimization of deep-infiltrating lesions (DIEs) [3], a severe type of lesion causing extensive scarring, that was observed solely in dienogest-treated patients [4]. This result was supported by a 2017 study that further confirmed dienogest’s baseline efficacy for reducing DIEs [10]. Thus, the results of these two studies suggest dienogest as a superior option, particularly for patients with DIEs that are characteristic of more severe endometriosis stages.
These studies strongly point towards the implication that dienogest could be more effective than COCP treatment at reducing and minimizing endometriomas on a histological level. However, given the limited number of recent studies, more research is required that directly compares dienogest to another treatment by evaluating details of endometrial cells. Still, these microscopic-level impacts are not the only concern—we must further investigate how those changes manifest in the body on a greater scale, such as in general fertility and overall quality of life.
Impact on Fertility
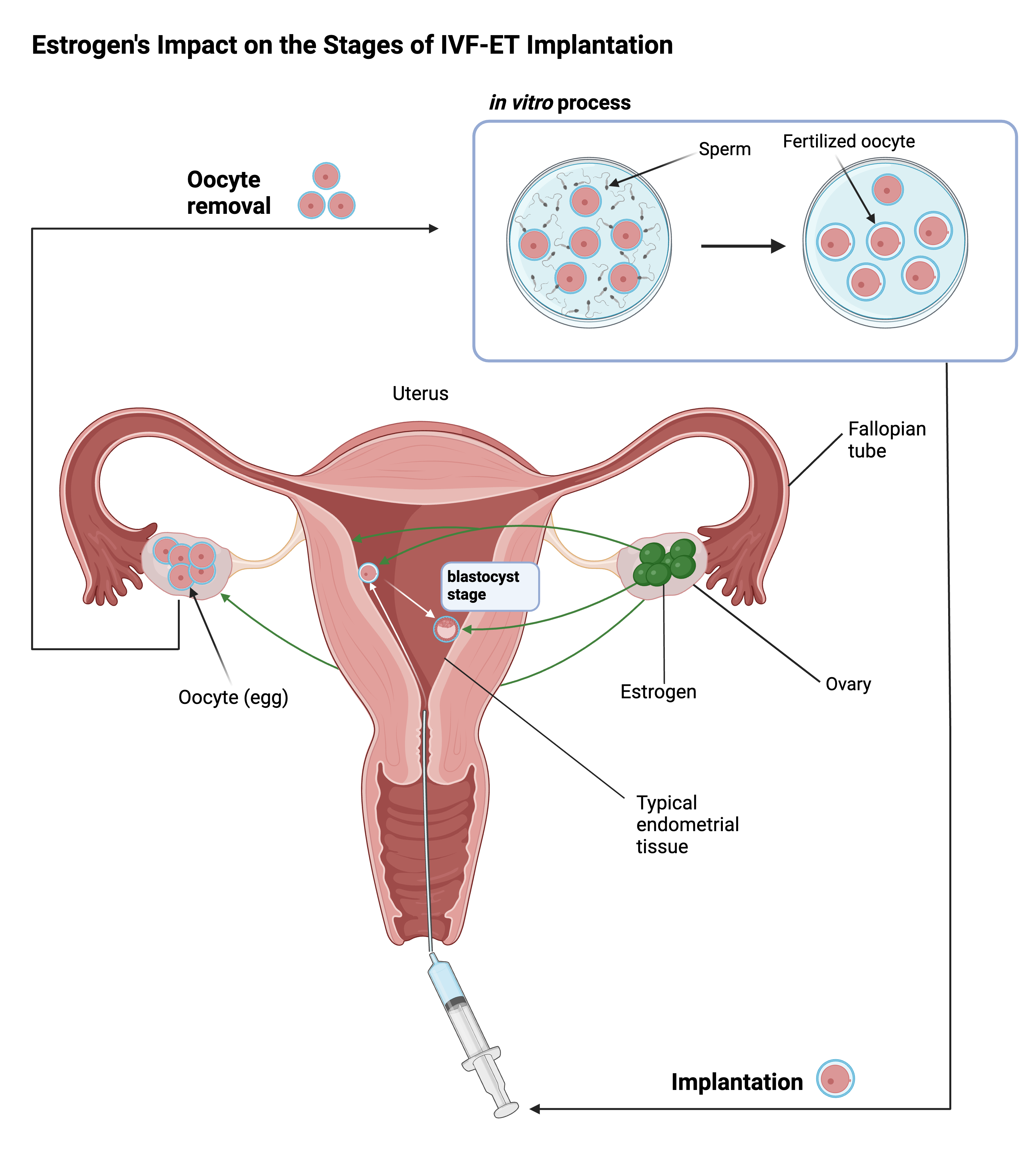
Endometriosis may harm fertility through various mechanisms: for example, built-up endometrial scar tissue can hinder the egg’s successful transportation and implantation in the uterus, and inflammation and oxidative damage may directly decrease the quality of the egg cell [6,7]. In-vitro fertilization and embryo transfer (IVF-ET) procedures are clinical procedures that improve chances of pregnancy through artificial methods of fertilization and embryo implantation. Thus, the success of IVF-ET procedures directly correlates to the efficacy of endometriosis drugs that rebalance female reproductive hormones in order to prevent and resolve endometrial damage. In a 2021 study conducted on IVF-ET patients at the Minia Infertility Research Unit in Egypt, researchers found that the discrepancy in fertility rate improvement between dienogest-treated and GnRH-treated patients was not significant enough to implicate either treatment as superior [6]. Furthermore, a 2019 study observed data from several fertility rate parameters—average number of retrieved oocytes, fertilized oocytes, mature growing follicles, and blastocysts—which were all lower in the dienogest treatment group compared to the GnRH treatment group [7]. The lower parameters indicate lower efficacy of IVF-ET procedures, which directly correlates to dienogest’s relatively poor impact on improving fertility compared to GnRH treatment. However, a 2021 study measured the number of viable ovarian follicles before and after GnRH and dienogest treatments and discovered significantly higher serum AMH levels (a hormone indicating ovarian health) in dienogest-treated patients than in patients given GnRH [8]. Thus, ovarian reserve as a measure of fertility was significantly more improved by dienogest, a result conflicting with the other studies that drew inconclusive comparisons between GnRH and dienogest. This contrast clearly indicates that further research is required to reach a conclusive stance on the superior fertility-improving treatment. Additionally, there currently exists a lack of research regarding the impact of long-term usage of either drug on fertility, which may be a major consideration for many IVF-ET-seeking patients.
While the previously-discussed studies which investigated cellular-level changes pointed to dienogest having a superior impact specifically on endometrial tissue histology, the results from these studies—regarding dienogest’s impact on resolving fertility issues caused by endometriosis—cast doubt on dienogest as a holistically more effective treatment. Given that existing research is widely limited to comparisons of dienogest vs. GnRH, future research must focus on also evaluating dienogest vs. COCP for fertility impact. Still, the aforementioned 2021 study explicitly pointed out a significantly lower cost of dienogest treatment compared to GnRH treatments [6]. It is crucial to consider the role that financial accessibility plays in patients’ preferred treatments, and thus important to not dismiss dienogest as a valid option. To continue creating a more holistic review of dienogest’s relative efficacy, we can look next at its impact on patients’ quality of life and reduced endometrial pain.
Endometriosis-Associated Pain and Quality of Life Improvement
This last section will compare the impact of dienogest treatment on improving self-assessed quality of life (QoL), compared to the impact of COCP and GnRH treatments. In a 2020 study conducted at multiple hospitals associated with the Iran University of Medical Sciences, researchers evaluated changes in chronic pelvic pain in patients recovering from laparoscopic excision of endometrial lesions as a measure of quality of life (QoL) improvement. They quantified the assessments by using the Visual Analogue Scale (VAS), an established method to appropriately standardize and compare subjectively reported measurements of pain in which a smaller VAS score indicates milder pain, and a larger score indicates more severe pain [3]. Results demonstrated that after a 6-month period, the difference between the average decrease in VAS score observed from patients in the dienogest treatment group and patients in the COCP treatment group was statistically insignificant [5]. However, both COCP and dienogest score changes were significantly greater than the placebo score change [5]. The most reasonable conclusion we can draw from this study is that COCP and dienogest are both effective in improving QoL, although at this time there is no conclusive difference in efficacy between these two treatments. Patients reported a lower level of endometriosis-associated pelvic pain when given either treatment than with no treatment at all.
This argument is supported by the results of a 2021 study conducted on patients with different stages of endometriosis severity at the Women’s Health Center at the American University of Beirut Medical Center, researchers evaluated patients’ self-reported QoL assessments after a 24-week period with either dienogest, COCP, or placebo treatments. They found that the average VAS scores of patients from both the dienogest and COCP treatment groups saw significant decreases from start to conclusion of the treatment period [3]. To corroborate these results, the researchers also used the Biberoglu and Behrman (B&B) scale which measures reduced chronic pelvic pain (CPP) and dysmenorrhea, and found that patients in both the dienogest and COCP groups saw significant reductions in both pain types, resulting in “none” or “mild” severity [3]. However, neither COCP nor dienogest had a statistically superior impact over the other on reducing these types of pain [3], a result which was similarly observed in a different 2021 study that also compared dienogest and COCP [9]. Thus, these studies strongly suggest that in regards to treating pelvic pain, neither dienogest nor COCP is conclusively superior to the other—although both remain effective options.
The current research supports both dienogest and COCP as viable options for easing endometriosis symptoms related to abdominal pain. However, given that there is no conclusively superior pain treatment at this point, further research may benefit from evaluating patients from the same stage of endometriosis in order to draw a stronger conclusion for a more limited scope. Furthermore, given that most studies compared dienogest to COCP when evaluating quality of life and reduced pain, future research should further evaluate dienogest in comparison to the GnRH treatment.
Conclusion
The studies evaluated in this review strongly implicate that dienogest is more effective than COCP treatment at reducing endometrial tissue on a histological level, yet it remains inconclusive whether or not dienogest is superior to GnRH treatment at improving fertility rates or superior to COCP at improving quality of life. It would be beneficial for future research to take a closer look at the specific cellular biochemical pathways that connect endometrial growth and estrogen production to these broader physiological factors of fertility and pelvic pain experienced by the patient. If some intermediate biomarker or receptor exists in these pathways that does not receive treatment from dienogest but does from COCP or GnRH, identifying it may allow us to predict how different cellular receptors (i.e. receptors targeted by dienogest vs. receptors targeted by GnRH) react to certain drugs and molecules in order to refine dienogest’s chemical targeting systems.
Whether or not dienogest is a more effective option for endometriosis treatment compared to GnRH or COCP may ultimately still depend on the unique user’s main concern. For instance, if a patient is primarily seeking to minimize endometrial pain, either dienogest or COCP may be of equal consideration. If they prefer to prioritize reducing endometrioma severity, dienogest may be a preferable option. Someone seeking improved fertility may find GnRH and dienogest both suitable, but the latter preferable given the relatively lower cost of dienogest treatments. Overall, more research on these various drugs is necessary not only to develop more holistically effective endometriosis treatment options but also to unearth clearer understandings of the many complex biological mechanisms that comprise uterine health and the female reproductive system.

About the Author: Annie Hu
Annie Hu is a third-year undergraduate student studying biotechnology at UC Davis, with a concentration in bioinformatics and an interest in gynecology that sparked at a young age. Her passion lies in studying the complex hormone pathways of the female reproductive system to better understand how to refine popular current treatments, such as oral contraceptives, in order to resolve common toxic side effects. She hopes that this paper sheds light on the continued uncertainties surrounding endometriosis, and that it helps readers recognize that progress starts with our willingness to listen to our mothers’, sisters’, and daughters’ diverse experiences as we look into these daunting, fascinating, and complex parts of the human body. Annie’s previous work in the Chen Lab at UC Irvine involved investigating protein modification and uterine blood vessel formation during pregnancy. Here at Davis, she conducts research as part of the UCD BioInnovation Group to develop a more sustainable and cost-accessible form of insulin derived from genetically engineered algae, and pushes to promote undergraduate resources and healthy collaboration amongst her peers as an organic chemistry tutor.
References
Adachi K, Takahashi K, Nakamura K, Otake A, Sasamoto N, Miyoshi Y, Shioji M, Yamamoto Y, Fujitani M, Wakimoto A, and others. Postoperative administration of dienogest for suppressing recurrence of disease and relieving pain in subjects with ovarian endometriomas. Gynecol Endocrinol. 2016 Aug [cited 2023 Apr 19];32(8):646-649. Available from https://www.tandfonline.com/doi/full/10.3109/09513590.2016.1147547 ?scroll=top&needAccess=true&role=tab&aria-labelledby=full-article. doi: 10.3109/09513590.2016.1147547.
Miyashita M, Koga K, Takamura M, Izumi G, Nagai M, Harada M, Hirata T, Hirota Y, Fujii T, Osuga Y. Dienogest reduces proliferation, aromatase expression and angiogenesis, and increases apoptosis in human endometriosis. Gynecol Endocrinol. 2014 Sep [cited 2023 Apr 24];30(9):644-8. Available from https://www.tandfonline.com/doi/abs/10.3109/09513590.2014.911279?journalCode=igy e20. doi: 10.3109/09513590.2014.911279.
El Taha L, Abu Musa A, Khalifeh D, Khalil A, Abbasi S, Nassif J. Efficacy of dienogest vs combined oral contraceptive on pain associated with endometriosis: Randomized clinical trial. Eur J Obstet Gynecol Reprod Biol. 2021 Dec [cited 2023 Apr 27];267:205-212. Available from https://www.sciencedirect.com/science/article/pii/S0301211521005261?via%3Dihub. doi: 10.1016/j.ejogrb.2021.10.029.
Piacenti I, Viscardi MF, Masciullo L, Sangiuliano C, Scaramuzzino S, Piccioni MG, Muzii L, Benedetti Panici P, Porpora MG. Dienogest versus continuous oral levonorgestrel/EE in patients with endometriosis: what's the best choice? Gynecol Endocrinol. 2021 May [cited 2023 Apr 27];37(5):471-475. Available from https://www.tandfonline.com/doi/full/10.1080/09513590.2021.1892632. doi: 10.1080/09513590.2021.1892632.
Mehdizadeh Kashi A, Niakan G, Ebrahimpour M, Allahqoli L, Hassanlouei B, Gitas G, Alkatout I. A randomized, double-blind, placebo-controlled pilot study of the comparative effects of dienogest and the combined oral contraceptive pill in women with endometriosis. Int J Gynaecol Obstet. 2022 Jan [cited 2023 Apr 24];156(1):124-132. Available from https://obgyn.onlinelibrary.wiley.com/doi/epdf/10.1002/ijgo.13677. doi: 10.1002/ijgo.13677.
Khalifa E, Mohammad H, Abdullah A, Abdel-Rasheed M, Khairy M, Hosni M. Role of suppression of endometriosis with progestins before IVF-ET: a non-inferiority randomized controlled trial. BMC Pregnancy Childbirth. 2021 Mar 30 [cited 2023 Apr 27];21(1):264. Available from https://bmcpregnancychildbirth.biomedcentral.com/articles/10.1186/s12884-021-03736-2. doi: 10.1186/s12884-021-03736-2.
Tamura H, Yoshida H, Kikuchi H, Josaki M, Mihara Y, Shirafuta Y, Shinagawa M, Tamura I, Taketani T, Takasaki A, and others. The clinical outcome of Dienogest treatment followed by in vitro fertilization and embryo transfer in infertile women with endometriosis. J Ovarian Res. 2019 Dec 12 [cited 2023 Apr 19];12(1):123. Available from https://ovarianresearch.biomedcentral.com/articles/10.1186/s13048-019-0597-y. doi: 10.1186/s13048-019-0597-y.
Muraoka A, Osuka S, Yabuki A, Bayasula, Yoshihara M, Tanaka H, Sonehara R, Miyake N, Murakami M, Yoshita S, and others. Impact of perioperative use of GnRH agonist or dienogest on ovarian reserve after cystectomy for endometriomas: a randomized controlled trial. Reprod Biol Endocrinol. 2021 Dec 6 [cited 2023 May 23];19(1):179. Available from https://rbej.biomedcentral.com/articles/10.1186/s12958-021-00866-2. doi: 10.1186/s12958-021-00866-2.
Niakan G, Rokhgireh S, Ebrahimpour M, Mehdizadeh Kashi A. Comparing the Effect of Dienogest and OCPS on Pain and Quality of Life in Women with Endometriosis: A Randomized, Double-Blind, Placebo-Controlled Trial. Arch Iran Med. 2021 Sep [cited 2023 May 23];24(9):670-677. Available from https://www.proquest.com/openview/cc931bfa6e5ef06c77aad9c327af763e/1?cbl=1057 83&pq-origsite=gscholar&parentSessionId=DBGm7T0wC7GWjrgfuTkqnl3SXUihDskMaBC8 bCPvtxU%3D. doi: 10.34172/aim.2021.96.
Yamanaka A, Hada T, Matsumoto T, Kanno K, Shirane A, Yanai S, Nakajima S, Ebisawa K, Ota Y, Andou M. Effect of dienogest on pain and ovarian endometrioma occurrence after laparoscopic resection of uterosacral ligaments with deep infiltrating endometriosis. Eur J Obstet Gynecol Reprod Biol. 2017 Sep [cited 2023 May 23];216:51-55. Available from https://www.sciencedirect.com/science/article/pii/S0301211517303469?via%3Dihub. doi: 10.1016/j.ejogrb.2017.07.014.
Williamson C, Bennett P. Basic Sciences in Obstetrics and Gynaecology. 4th ed. London (LDN): Elsevier Health Sciences; 2010 [accessed 2023 Aug 14]. https://ebookcentral.proquest.com/lib/uci/reader.action?docID=4683246.
- Cable JK, Grider MH. Physiology, Progesterone. StatPearls. 2023 May [cited 2023 Aug 2]. Available from https://www.ncbi.nlm.nih.gov/books/NBK558960/.
