
Advancing Bio-Based Alternatives to UV Gel Nail Products
Abstract
The beauty industry has witnessed a growing concern regarding the environmental impact of conventional nail polish formulations, particularly, those containing fossil fuel-based polymers and harmful chemicals. The search for eco-friendly alternatives has led to the exploration of bio-based polymers derived from natural resources, such as plant oils, plant starches, and bio-based monomers. This review focuses on six bio-based polymer alternatives that have shown promise in gel nail polish formulations. These include polymers derived from plant products, such as soybean oil, castor oil, citric acid, and cassava starch. This review also highlights the different synthetic and formulation methods that enable the transformation of these materials into functional coatings. Varying these parameters can influence desirable gel polish characteristics, such as durability, finish, and resistance to environmental factors. Lastly, this review will discuss various testing methods employed to assess bio-based UV-cured coatings compared to their industry standards. In short, this review underscores the growing importance of bio-based UV-cured gel nail polishes as sustainable alternatives to commercial products. By analyzing their synthetic and formulation methods, we can provide a bio-based nail product with full industry potential.
Introduction
Plastics are employed in a wide range of items in the cosmetics industry, ranging from packaging, tools, and even product composition. Though its durability and affordability makes it useful for everyday items, its resistance to wear, heat, and chemicals makes plastic one of the least sustainable materials on the planet.
Similar to the cosmetics industry, the nail industry has also seen a growing effort to incorporate environmentally friendly materials and methods. Though the nail industry is arguably one of the least sustainable branches of cosmetics today due to nearly all nail products consisting of petrochemicals, or fossil fuel-derived materials, sustainability in nail cosmetics is a topic yet to be explored in depth. Nonetheless, the polymeric formulation of gel nail polish provides its resistance to scratches, chips, peels, and chemicals [1]. Similar to other plastics applications, the nail cosmetics industry is developing novel formulations of organic coatings using bio-renewable, natural, materials such as plant oils and starches, in effort to replace their plastic-based counterparts [1].
Studies in this review explore the potential of bio-based materials, formulation strategies, and testing methods that can ultimately reduce reliance on fossil fuels and enable the transition toward a more sustainable cosmetics industry. In short, this literature review will showcase the synthesis, formulation, and testing of bio-based UV-curable coatings as sustainable alternatives to conventional gel nail polishes.
Overview of UV-Cured Gel Nail Polish

Gel nail polishes are a popular branch of nail cosmetics that, compared to traditional nail polish, are low in volatile organic compounds (VOCs), cured by exposure to ultraviolet (UV) radiation, and last on the nail plates for approximately 2 weeks [2,3]. Application typically consists of coating the nail plate with UV-curable nail polish, then curing the nails under a specialized UV or UV-LED lamp. This results in a shiny, durable, plastic-like coating over the nail.
Gel polish formulas consist of several chemical components, including binders, solvents, photoinitiators, pigments, and other additives. Binders are the base material of gel polish, consisting of plastic-like compounds that create a durable, plastic-like coating on the nail. Solvents, also known as diluents, are typically the second largest component of gel polish formulations. Solvents tweak the viscosity of the final product and can also alter the physical properties of the polish [3]. Photoinitiators are molecules that, when exposed to UV light, act as a catalyst in polymerization reactions. This is what allows gel polishes and other coatings to cure under exposure to UV lamps. Pigments are purely cosmetic additives that control the final color and look of the polish, like dyes, glitters, or minerals. Finally, additives are chemicals that do not interfere with the performance of the gel but improve factors like cure efficiency, shelf life, and pigment dispersion [1,3].
Synthesis and Formulation
Raw Materials
The primary ingredient in UV-cured organic coatings are polymers, which are large molecular structures consisting of several long carbon chains attached to each other. Common polymeric materials in the organic coating industry include polyurethane acrylates, polyester acrylates, epoxy acrylates, and methacrylates [2]. These materials have been implemented in a wide array of UV-cured organic coating applications, spanning the automotive, aerospace, 3D printing, and coating industries, and are derived from fossil fuels and oils. To develop alternatives to petroleum-derived products, researchers utilize the molecular similarities between traditional fossil fuels and plant-based oils. The studies discussed in this review develop vegetable-derived polymers including castor oil [4,5], soybean oil [1,3], citric acid [6], and cassava starch [7].
UV-Cure Chemistry
Plant oils are a category of organic molecules called triglycerides, consisting of three long, complex carbon branches stemming from a single carbon atom. These carbon chains can house a multitude of functional groups, wherein each group and its position can affect the oil’s physical and chemical properties. In their raw form, plant oils are considered monomers, or the individual units that make up a polymer. To create a molecule suitable for UV curing, plant oils are chemically modified to strengthen their polymerization capability [8].
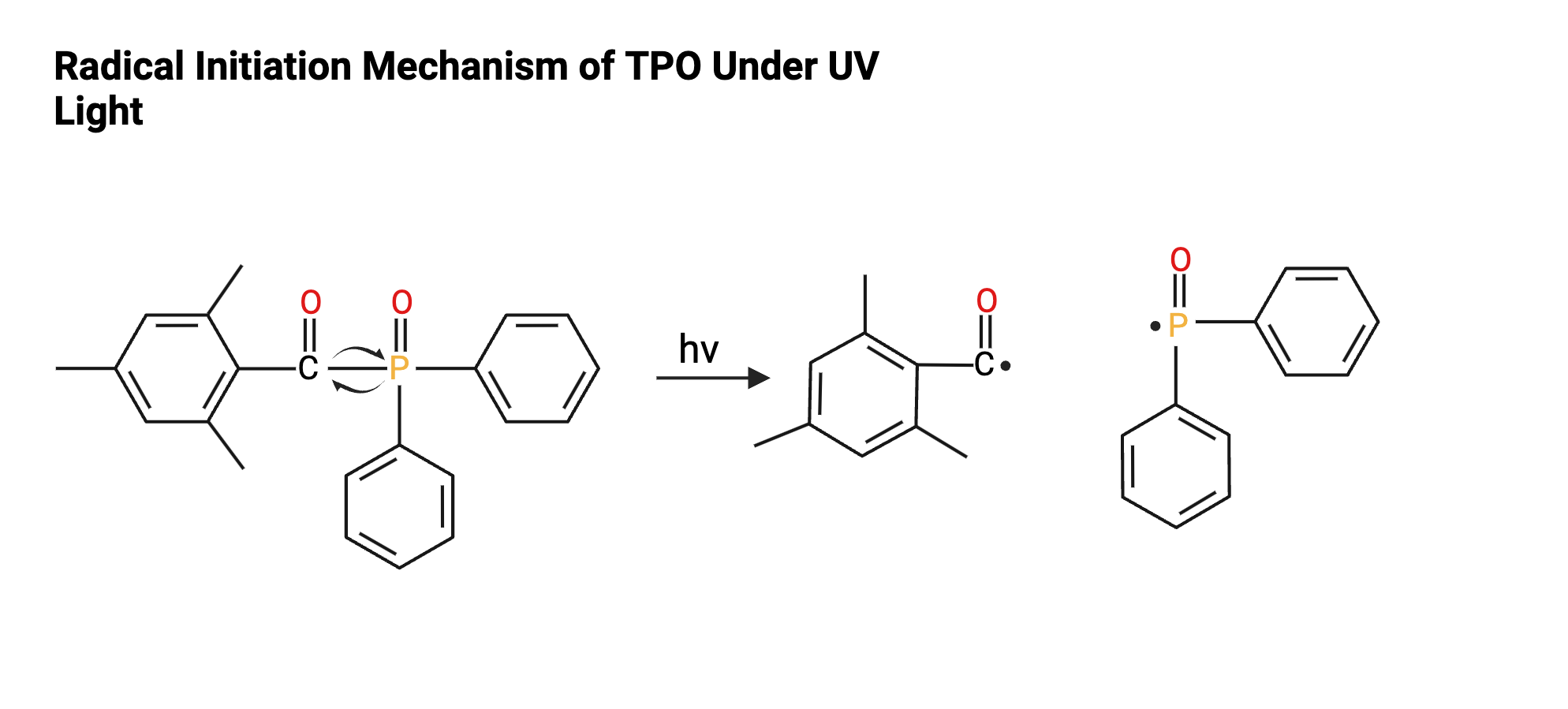
As mentioned previously, photoinitiators are compounds that begin a gel polish’s curing process once exposed to UV light. When excited, the photoinitiator molecule undergoes a cleavage reaction, generating a free radical [2]. Free radicals are highly reactive unpaired electrons that are crucial catalysts in curing reactions. When radicals interact with molecules with functional groups such as alkenes (vinyls) or acrylates, those molecules undergo a radical chain polymerization mechanism [8]. Polymerization is the process by which individual monomers are chemically bonded together to form a large, repeating structure, or a polymer. Through this process, the product is converted from a gel-like liquid to a cured solid material [2].
Synthesis
In these studies, the bio-based materials are processed using one of two synthetic pathways. The first synthetic pathway adds functional groups to the structure to make it more suitable for polymerization, typically via epoxidation, hydrogenation, esterification, or acrylation reactions. [8]. The addition of such functional groups allow the oil molecules to attach to one another via polymerization reactions under UV light [8]. In the case of soybean oil, the double bonds along its carbon chain leave it susceptible to acrylation and epoxidation [1,3].


For the additive synthetic pathways, including plant-derived compounds like citric acid and cassava starch, producing a final polymer is much more indirect. For citric acid, its structure already resembles that of a triglyceride wherein three carboxyl groups neighbor a single carbon atom. With the addition of a non-bio-based polymer, such as polyethylene glycol and several other functional molecules, the three carbon branches of the citric acid molecule can be expanded into a coating with both rigid and elastic properties [6].

For the cassava starch formulation, the starch itself was considered an additive, while the binder consisted of another polymeric material. Essentially, this starch, also known as cellulose, acts as a hardening agent in the final coating rather than the binding agent. The bio-polymer used in this study is a waterborne polyurethane-acrylate derived from natural rubber [7].

Formulation
As stated, UV-cured organic coatings consist of base polymers, photoinitiators, solvents, and other additives [1,3]. Once the bio-based polymers are prepared, several factors need to be considered in selecting additives. Specifically, scientists may choose not to use solvents or photoinitiators with high skin irritancy, strong odor, or environmental toxicity [1]. While the silicone-castor oil and cassava starch studies formulated their coatings from polymers alone, other studies used common solvents in their formulas, including pentaerythritol tetracrylate (PETA) [5], tri(propylene glycol) diacrylate (TPGDA) [3,6], and ethyl acetate [1]. Given that TPGDA and PETA are environmental toxins and irritants, they are not ideal solvent choices for a product meant for topical use [3]. One experiment studying soybean oil-derived polymers implemented ethyl acetate, a common solvent in chemical synthesis, as their main solvent [1].
One very popular photoinitiator, TPO, was used in studies concerning castor oil derivatives and soybean oil derivatives. This resulted in the cure time for the castor oil formula to be 30 seconds [5], while that for the soybean oil formulas was approximately 60 seconds [1,3]. While 30 seconds and 60 seconds are within the range for a traditional gel polish, there are most likely other factors, such as structural differences between the polymers, that account for the cure time differences between the soybean oil and castor oil derivatives.
Testing
After all of the bio-based UV-cured coatings have been formulated, researchers often test their physical characteristics against an ‘industry standard’ gel product to see how they compare. Physical characteristics, like durability and adhesion of the finalized gel polishes, can be analyzed using the gloss test, pencil hardness test, and the cross-hatch adhesion test [1]. This review uses the popular gel polish system, Shellac Combo by CND, as the benchmark for all tests. The test results are used to quantifiably compare and contrast each of the formulas in this review.
Cure Time
Cure time is defined as the time required for a coating to fully transform from a liquid state to a solid state. For the benefit of consumers, it is desirable for a gel nail polish to dry, or cure, as fast as possible. Therefore, researchers seek to adjust their formulations to minimize cure time. The time for a coating to fully cure can be measured with Fourier Transform Infrared (FT-IR) spectroscopy, where researchers analyze spectroscopic changes before, during, and after curing [4]. Based on the consumer standard gel polish by CND, the ideal time for gel polish to fully cure should be approximately 1-2 minutes [1].
As mentioned previously, both soybean oil derivative and cassava starch formula cured in approximately 60 seconds [1,3,7]. Given that all three of these formulations used the industry standard photoinitiator, TPO, it is reasonable that they have similar cure times. Other formulations, like the two castor oil derivatives, had much quicker cure times, at 30 to 40 seconds [4,5]. Though they used different photoinitiators, the decreased cure times may be the result of a castor oil base.
Gloss
Gloss is a very important characteristic when discussing any type of paint or coating. Though a seemingly qualitative criterion, gloss is often measured quantitatively with a gloss meter. A gloss meter is an instrument that emits a beam of light onto a surface, then reports the amount of light reflected at a particular angle. According to measurements of the CND benchmark, the ideal gloss meter reading for gel polish is 85 gloss units (GU) or greater, where glossiness increases with numerical value [1].
Of the studies that included gloss tests, three of the four passed the desired gloss measurement. The citric acid derived coating had a gloss reading of exactly 85 GU [6], while the soybean oil-derived polishes surpassed this, with readings of 88 and 92 GU [1,3]. The cassava starch-derived coating had a gloss reading of only 46.2 GU [7]. This low gloss value is attributed to the fact that the cassava starch was used as a strengthening additive rather than the binding material in this formulation. In other words, starch naturally has a much less glossy appearance compared to its synthetic counterparts. Additionally, neither castor oil derivative studies included gloss readings at all, most likely because their applications in industrial coatings do not require a high gloss formula.
Pencil Hardness
The pencil hardness test is a simple way to indicate scratch resistance in a material [1]. As mentioned in the overview section, increased durability is a desirable characteristic of gel nail formulas. This test is performed by abrading the surface of the cured coating with different hardnesses of pencils, ranging from 9B to 9H [1]. By identifying the softest pencil able to scratch the surface of the paint, researchers can determine the hardness of the polish. According to consumers, the ideal pencil hardness value for a gel nail polish is between 2H and 6H [1,3].

Scratch resistance was perhaps the most variable metric within these studies. The formulas scratched by the highest pencil hardnesses, 9H and 6H, were both castor oil derivatives [4,5]. This hardness characteristic can be attributed to the unique molecular structure of castor oil, which contains unsaturated alkene bonds and hydroxyl content [4]. Furthermore, increased hydrogen bonding in a molecule in the urethane-citric acid-derived formula results in an equally high hardness value, 6H [6]. In contrast, the soybean oil-derived formulas had significantly decreased pencil hardness values, at 3H [3] and 2H [1]. This increased scratch susceptibility is most likely due to the close carbon-carbon double bonding in the soybean oil structures [1]. This results in higher rigidity in the final product, as opposed to elasticity, which decreases pencil hardness.
Adhesion

A gel polish must adhere strongly to the wearer’s nail plate to survive a 2 to 3 week wear time [3]. The level of adhesion of a coating on a surface can be evaluated using the cross-cut or cross-hatch adhesion test. This test is performed by painting and curing a coating onto a substrate, or test material, then carving a cross-hatch pattern into the sample with a utility knife [6]. An adhesive tape or stamp is applied to the cross-cut material and is rapidly ripped off to remove the paint underneath. This test measures the percentage of material removed by the tape, where ratings range from 5B, or 0% of material removed, to 0B, or greater than 65% of material removed [6]. Essentially, the amount of product removed during this test can determine whether or not a paint or coating is adhesive to the substrate. Given the properties of the benchmark standard gel nail polish, the ideal cross-hatch adhesion measurement is 5B [1].
Two of the six bio-based formulas had perfect 5B cross-cut adhesion test ratings – the soybean oil and silicone-castor oil derivatives. The strong adhesion of the polyester-soybean oil derivative can be attributed to the high hydroxyl content, as that can induce intermolecular interactions between the coating and the substrate [1]. In contrast, the strong adhesion of the silicone-castor oil-derived formula is heavily attributed to the ratio of acrylate to thiol groups in the formula [4]. Furthermore, other formulas, including the pure castor oil derivative, had an adhesive rating of 4B [5]. Though this is not the ideal rating, researchers identified the thermal
stability additive, B-215, to influence adhesive character. As B-215 content increased, the bulky carboxyl groups were shown to limit polymer mobility, therefore improving adhesion between the coating and substrate [5]. Similarly, the citric-acid derived polymers had lesser adhesion, at 4B, due to less overlap, or crosslinking density, between each other [6].
Overall, optical and mechanical tests were performed for cure time, gloss, scratch resistance, and adhesion to determine what factors, reagents, or raw materials result in a desirable novel gel nail polish formula. According to the industry standard, this requires the bio-based gel polish to cure in approximately one minute, have gloss meter readings greater than 85 gloss units, have a pencil hardness rating between 2H and 6H, and have a cross-cut adhesion value of 5B [1,3]. Given these criteria, nearly all of the formulas discussed in this review have the potential to be manufactured and sold as bio-based gel nail polishes.
Conclusion
This review explores various bio-based materials and their applications in the development of UV-cured coatings. Focusing on the synthetic method, formulation, and testing of these coatings, these studies conclude their potential as sustainable alternatives to plastic-based gel nail polishes. By comparing the mechanical characteristics of finished coatings derived from castor oil, citric acid, cassava starch, and soybean oil, it is shown that the polyester-soybean oil derivative has the greatest potential to replace fossil fuel-based commercial gel products. This formulation developed by Dr. Zareanshahraki included crucial components such as ethyl acetate, an environmentally neutral solvent, and TPO, the industry-standard photoinitiator [1]. According to the testing parameters, the soybean oil gel polish excelled in cure time, gloss, scratch resistance, and adhesion.
Nonetheless, there are several gaps that these studies overlook. Several studies did mention and measure their material’s thermal stability, or the temperature required to decompose or degrade the cured coating [6]. Though useful to know if subjecting the coating to a high-temperature environment, this is not very useful for gel nail polishes, as consumers of this product would most likely feel high physiological discomfort before reaching this threshold. A more useful thermal test would be to measure the temperature change of the gel polish during the curing process under UV radiation. Because the UV-curing reaction is exothermic, many consumers feel discomfort due to the increased temperature on their nail beds [2]. Therefore, researchers should strive to formulate a gel polish with a relatively low curing temperature.
Most notably, the majority of the bio-based UV-cured coating studies in this review are meant for other applications, such as wood coatings and 3D printing, as opposed to gel nail polish [2,4,5]. Though the synthetic, formulation, and testing processes are similar, these formulations are not designed for cosmetic applications, much less for human use. Although the two castor oil derivatives and the citric acid derivative are effective products according to the testing criteria, it is still unknown if they are safe for human use. Therefore, additional theoretical or practical testing of these coatings is still required for them to be viable consumer products.
Overall, current research concerning bio-based UV-cured organic coatings presents a promising pathway toward more sustainable and environmentally-friendly nail products. While gel nail systems make outstandingly durable and long-lasting manicures, traditional gel polish materials are extremely unsustainable. Bio-based alternatives not only display comparable performance characteristics to commercial gel polishes but also contribute to reducing the industry’s carbon footprint and dependence on fossil fuels. In short, this research provides a future toward reducing the environmental impact of the beauty industry overall.

About the Author: Jenna Khun
Jenna Khun is a 4th year Chemistry major minoring in Political Science at UC Davis. For the past 5 years, she has worked as a freelance nail artist specializing in the Gel-X nail extension application system. Ever since high school, she has wanted to explore the intersections between science, society, and art. She has a vast array of interests in the field of chemistry, ranging anywhere from research and development of cosmetic products, to organic synthesis, and even quantum mechanics. Currently, she is an undergraduate researcher in Dr. Selina Wang’s food chemistry lab, a group dedicated to ensuring food quality, authenticity, and sustainability. While also working as a laboratory assistant in UCD’s organic chemistry teaching labs, Jenna currently sees her future in both industry and academia.
References
- Zareanshahraki, Forough, and Vijay Mannari. 2021. “Optimizing the Performance of Sustainable Nail Gel Compositions Using a Mixture Experimental Design Methodology.” Prog Org Coat. 153 (4): 106168.
- Dvorchak, Michael J, and Melanie L Clouser. 2021. “UV Curing of Nail Gels by Light Emitting Diode (LED) and Fluorescent (FL) Light Sources.” Surf Sci Adhes Cosmet. 3 (March): 73–107.
- Zareanshahraki, Forough, and Vijay Mannari. 2018. “‘Green’ UV-LED Gel Nail Polishes from Bio-Based Materials.” Int J Cosmet Sci. 40 (6): 555–64.
- Cheng, Fei, Yunxin Fan, Na He, Yan Song, Jianbin Shen, Zhangshui Gong, Xiaomei Tong, and Xiongfa Yang. 2022. “Castor Oil Based High Transparent UV Cured Silicone Modified Polyurethane Acrylate Coatings with Outstanding Tensile Strength and Good Chemical Resistance.” Prog Org Coat. 163 (22): 106624.
- Liang, Bin, Renpu Li, Chaoqun Zhang, Zhuohong Yang, and Teng Yuan. 2019. “Synthesis and Characterization of a Novel Tri-Functional Bio-Based Methacrylate Prepolymer from Castor Oil and Its Application in UV-Curable Coatings.” Ind Crops Prod. 135 (19): 170–78.
- Maity, Debarati, Rahul Tade, and Anagha S. Sabnis. 2023. “Development of Bio-Based Polyester-Urethane-Acrylate (PUA) from Citric Acid for UV-Curable Coatings.” J Coat Technol Res. 20 (2).
- Tsupphayakorn‐aek, Phanthanyaphon, Anutida Suwan, Thawanrat Chaisit, Tulyapong Tulyapitak, Pranee Phinyocheep, Jean‐Francois Pilard, Nitinart Saetung, and Anuwat Saetung. 2023. “A New UV-Curable Biodegradable Waterborne Polyurethane-Acrylate Based on Natural Rubber Blended with Cassava Starch.” J Appl Polym Sci. 140 (17).
- Zareanshahraki, Forough. “Development of Sustainable Polymers as a Platform for Advanced UV-Curable Coatings.” PhD diss. Eastern Michigan University, 2021.
